List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Sc scandium
Sc - scandium - TRANSITION METAL
Scandium is a metallic chemical element belonging to the group of rare earths. Of series 3, it is characterized by an atomic number of 21 and an atomic weight of 44.95. In terms of physical properties, scandium is distinguished by a melting point of 1541°C and a boiling point of 2832°C. It is silvery white and occurs as a soft metal.
scandium makes up about 0.5% of Earth's rocks. It is mainly found in the form of apatite and silicates. Besides being found in some natural sources, it is produced mainly from Scandinavian ore.
scandium has mainly industrial uses. Indeed, this chemical element is used for the manufacture of metal alloys, in particular those intended for engineering and aerospace. These alloys are particularly known for their resistance to corrosion, their lightness and their resilience at high temperatures.
scandium also has interesting properties in the field of lighting. Indeed, this chemical element is used to produce high intensity discharge lamps that can be used for industrial lighting and cinema. In addition, scandium is also used in the manufacture of batteries for solar energy and in the production of catalysts which are used to treat air and water.
scandium makes up about 0.5% of Earth's rocks. It is mainly found in the form of apatite and silicates. Besides being found in some natural sources, it is produced mainly from Scandinavian ore.
scandium has mainly industrial uses. Indeed, this chemical element is used for the manufacture of metal alloys, in particular those intended for engineering and aerospace. These alloys are particularly known for their resistance to corrosion, their lightness and their resilience at high temperatures.
scandium also has interesting properties in the field of lighting. Indeed, this chemical element is used to produce high intensity discharge lamps that can be used for industrial lighting and cinema. In addition, scandium is also used in the manufacture of batteries for solar energy and in the production of catalysts which are used to treat air and water.
Synthetic
Radioactive
Liquid
Gaseous
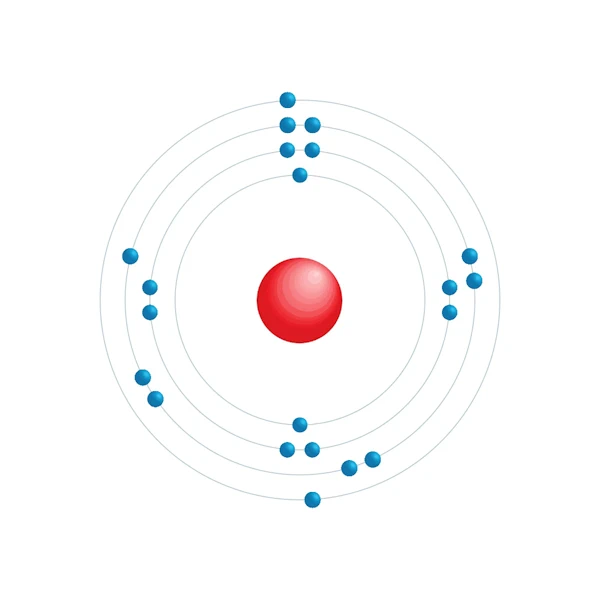
Electronic configuration diagram
| Name | scandium |
| Number | 21 |
| Atomic | 44.955908 |
| Symbol | Sc |
| Fusion | 1539 |
| Boiling | 2832 |
| Density | 2.989 |
| Period | 4 |
| Group | 3 |
| Discovery | 1878 Nilson |
| Abundance | 22 |
| Radius | 2.1 |
| Electronegativity | 1.36 |
| Ionization | 6.5615 |
| Number of isotopes | 15 |
| Electronic configuration | [Ar] 3d1 4s2 |
| Oxidation states | 1,2,3 |
| Electron by energy level | 2,8,9,2 |
| Mineral | Hardness | Density |
| Allendeite | 4.84 | |
| Arrojadite-(SrFe) | ||
| Bazzite | 6.50 / 6.50 | 2.80 |
| Cascandite | 4.50 / 5.50 | 3.01 |
| Davisite | ||
| Dissakisite-(La) | 6.50 / 7.00 | 3.79 |
| Eringaite | 3.65 | |
| Heftetjernite | 4.50 / 4.50 | |
| Jervisite | 6.00 / 7.00 | 3.22 |
| Juonniite | 4.00 / 4.50 | 2.43 |
| Kangite | ||
| Kolbeckite | 5.00 / 5.00 | 2.44 |
| Kristiansenite | 5.50 / 6.00 | 3.30 |
| Lakargiite | 8.00 / 8.50 | 4.59 |
| Magbasite | 5.00 / 5.00 | 3.41 |
| Oftedalite | 6.00 / 6.00 | 2.61 |
| Panguite | 3.75 | |
| Pretulite | 5.00 / 5.00 | 3.71 |
| Samarskite-(Yb) | 5.00 / 6.00 | 7.03 |
| Scandiobabingtonite | 6.00 / 6.00 | 3.24 |
| Stavelotite-(La) | 4.49 | |
| Thortveitite | 6.50 / 6.50 | 3.50 |
| Warkite |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





