List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Ir Iridium
Ir - Iridium - TRANSITION METAL
Synthetic
Radioactive
Liquid
Gaseous
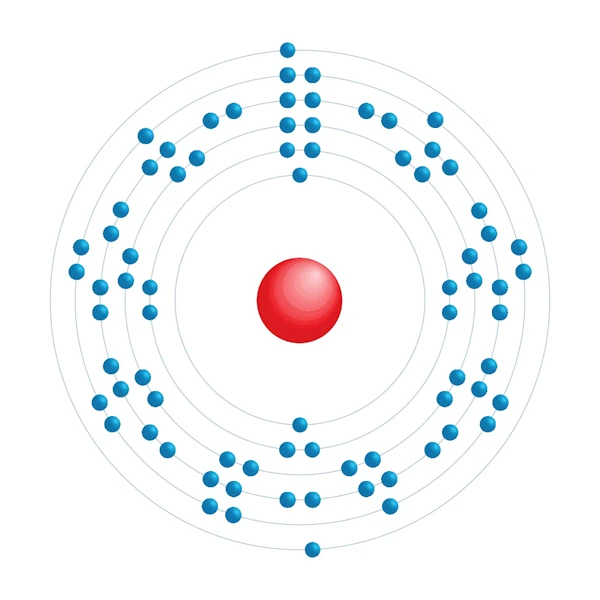
Electronic configuration diagram
| Name | Iridium |
| Number | 77 |
| Atomic | 192.217 |
| Symbol | Ir |
| Fusion | 2410 |
| Boiling | 4130 |
| Density | 22.56 |
| Period | 6 |
| Group | 9 |
| Discovery | 1804 Tennant |
| Abundance | 0.001 |
| Radius | 1.9 |
| Electronegativity | 2.2 |
| Ionization | 8.967 |
| Number of isotopes | 25 |
| Electronic configuration | [Xe] 4f14 5d7 6s2 |
| Oxidation states | -3,-1,1,2,3,4,5,6 |
| Electron by energy level | 2,8,18,32,15,2 |
| Mineral | Hardness | Density |
| Beta - iridisite | ||
| Bowieite | 7.00 / 7.00 | |
| Changchengite | 3.50 / 3.50 | 11.96 |
| Chengdeite | 5.00 / 5.00 | 19.30 |
| Cuproiridsite | 5.00 / 6.00 | 7.24 |
| Ferhodsite | ||
| Ferrorhodsite | 4.50 / 4.50 | 5.71 |
| Gaotaiite | 3.00 / 3.00 | 9.97 |
| Garutiite | 11.33 | |
| Hexaferrum | 6.00 / 7.00 | 10.69 |
| Hexamolybdenum | 11.90 | |
| Inaglyite | 5.50 / 5.50 | |
| Irarsite | 6.50 / 6.50 | 11.00 |
| Iridarsenite | 5.00 / 5.50 | 10.90 |
| Iridium | 6.00 / 7.00 | 22.60 |
| Kashinite | 7.50 / 7.50 | 9.10 |
| Kingstonite | 6.00 / 6.00 | 7.52 |
| Konderite | 5.50 / 5.50 | 4.73 |
| Malanite | 5.00 / 5.00 | 7.40 |
| Mayingite | 4.00 / 4.00 | 12.72 |
| Osmium | 6.00 / 7.00 | 19.00 |
| Rutheniridosmine | 6.00 / 7.00 | 21.00 |
| Ruthenium | 6.50 / 6.50 | 12.20 |
| Shuangfengite | 3.00 / 3.00 | 10.14 |
| Tolovkite | 7.50 / 7.50 | 10.50 |
| Xingzhongite | 6.00 / 6.00 | 6.64 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





