List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
Ba - Barium - ALKALINE EARTH METAL
Barium is a chemical element with atomic number 56 and symbol Ba. It is a heavy metal, mainly produced during the merger of supernovae and neutron stars. It is located in goup 8A of the Periodic Table of Elements.
barium is a shiny metal, is quite soft, and is silvery-white when freshly reduced. It has a density of 3.5 g/cm3 and melts at 725°C. It is soluble in sulfuric acid, but not in nitric acid or hydrochloric acid.
barium is used in a variety of applications, including in industry for melting metals, in the alloy industry, and as a catalyst in chemical reactions. It is also used as a material for making small metal parts and dyes for textiles.
In medicine, barium is used as a contrast agent for radiological examinations and for the treatment of kidney and liver diseases. It is also used as a medicine for osteoporosis.
barium has many properties that make it a useful material for many applications. It is non-toxic, non-corrosive and non-flammable. It has a strong radiation absorption capacity and is easy to work with. It is often used for melting metals and alloys. Finally, it is also an excellent catalyst for chemical reactions.
barium is a shiny metal, is quite soft, and is silvery-white when freshly reduced. It has a density of 3.5 g/cm3 and melts at 725°C. It is soluble in sulfuric acid, but not in nitric acid or hydrochloric acid.
barium is used in a variety of applications, including in industry for melting metals, in the alloy industry, and as a catalyst in chemical reactions. It is also used as a material for making small metal parts and dyes for textiles.
In medicine, barium is used as a contrast agent for radiological examinations and for the treatment of kidney and liver diseases. It is also used as a medicine for osteoporosis.
barium has many properties that make it a useful material for many applications. It is non-toxic, non-corrosive and non-flammable. It has a strong radiation absorption capacity and is easy to work with. It is often used for melting metals and alloys. Finally, it is also an excellent catalyst for chemical reactions.
Synthetic
Radioactive
Liquid
Gaseous
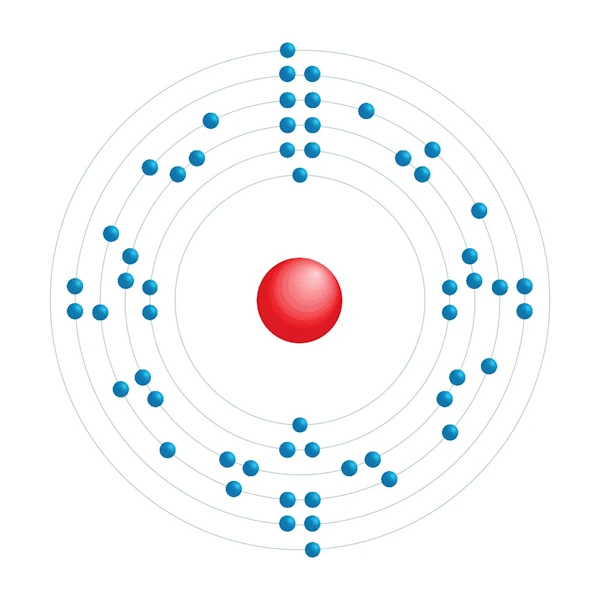
Electronic configuration diagram
| Name | Barium |
| Number | 56 |
| Atomic | 137.327 |
| Symbol | Ba |
| Fusion | 725 |
| Boiling | 1640 |
| Density | 3.594 |
| Period | 6 |
| Group | 2 |
| Discovery | 1808 Davy |
| Abundance | 425 |
| Radius | 2.8 |
| Electronegativity | 0.89 |
| Ionization | 5.2117 |
| Number of isotopes | 25 |
| Electronic configuration | [Xe] 6s2 |
| Oxidation states | 2 |
| Electron by energy level | 2,8,18,18,8,2 |
| Mineral | Hardness | Density |
| Alforsite | 5.00 / 5.00 | 4.73 |
| Alsakharovite-Zn | 5.00 / 5.00 | 2.90 |
| Alstonite | 4.00 / 4.50 | 3.69 |
| Anandite | 3.00 / 4.00 | 3.94 |
| Andrémeyerite | 5.50 / 5.50 | 4.15 |
| Ankangite | 6.50 / 6.50 | 4.44 |
| Aradite | ||
| Arctite | 5.00 / 5.00 | 3.11 |
| Armenite | 7.50 / 7.50 | 2.76 |
| Arrojadite-(BaFe) | 3.54 | |
| Arrojadite-(BaNa) | ||
| Arrojadite-(SrFe) | ||
| Arsenoflorencite-(Nd) | 3.50 / 3.50 | 4.05 |
| Arsenogorceixite | 4.00 / 4.00 | 3.65 |
| Arsenogoyazite | 4.00 / 4.00 | 3.33 |
| Asselbornite | 3.00 / 3.00 | |
| Babefphite | 3.50 / 4.00 | 4.31 |
| Bafertisite | 5.00 / 5.00 | 3.96 |
| Balipholite | 5.00 / 5.50 | 3.33 |
| Banalsite | 6.00 / 6.00 | 3.07 |
| Baotite | 6.00 / 6.00 | 4.42 |
| Bario-olgite | 4.00 / 4.50 | 4.00 |
| Bario-orthojoaquinite | 5.00 / 5.50 | 3.96 |
| Barioferrite | 5.31 | |
| Bariomicrolite | 4.50 / 5.00 | 5.68 |
| Barioperovskite | ||
| Bariopharmacoalumite | 3.50 / 3.50 | 2.58 |
| Bariopharmacosiderite | 3.00 / 3.00 | 3.07 |
| Bariopyrochlore | 4.50 / 5.00 | 4.00 |
| Bariosincosite | 3.00 / 3.00 | |
| Barium-zinc alumopharmocosiderite | 2.50 / 2.50 | |
| Barylite | 6.00 / 7.00 | 4.02 |
| Baryte | 3.00 / 3.50 | 4.47 |
| Barytocalcite | 4.00 / 4.00 | 3.64 |
| Barytolamprophyllite | 2.00 / 3.00 | 3.62 |
| Batiferrite | 6.00 / 6.00 | |
| Batisite | 5.90 / 5.90 | 3.43 |
| Batisivite | 7.00 / 7.00 | 4.62 |
| Bauranoite | 5.00 / 5.00 | 5.28 |
| Bavsiite | ||
| Bazirite | 6.00 / 6.50 | 3.82 |
| Belkovite | 6.00 / 7.00 | 4.16 |
| Bellbergite | 5.00 / 5.00 | 2.20 |
| Benauite | 3.50 / 3.50 | 3.65 |
| Benitoite | 6.00 / 6.50 | 3.60 |
| Benstonite | 3.00 / 4.00 | 3.60 |
| Bergenite | 2.00 / 3.00 | 4.10 |
| Bigcreekite | 2.00 / 3.00 | 2.66 |
| Billietite | 5.27 | |
| Biraite-(Ce) | 5.00 / 5.00 | 4.76 |
| Bjarebyite | 4.00 / 4.00 | 4.02 |
| Bobshannonite | 4.00 / 4.00 | 3.79 |
| Bobtraillite | 5.50 / 5.50 | 3.16 |
| Bornemanite | 3.50 / 4.00 | 3.47 |
| Bredigite | 3.40 | |
| Brewsterite-Ba | 5.00 / 5.00 | 2.45 |
| Brewsterite-Sr | 5.00 / 5.00 | 2.45 |
| Burbankite | 3.50 / 3.50 | 3.50 |
| Burovaite-Ca | 2.73 | |
| Bussenite | 4.00 / 4.00 | 3.63 |
| Byelorussite-(Ce) | 5.50 / 6.00 | 3.92 |
| Bykovaite | 3.00 / 3.00 | 2.98 |
| Byzantievite | 4.50 / 5.00 | 4.25 |
| Bøgvadite | 4.00 / 4.00 | 3.85 |
| Calciouranoite | 4.00 / 4.00 | 4.62 |
| Cámaraite | ||
| Canosioite | 4.94 | |
| Cappelenite-(Y) | 6.00 / 6.00 | 4.40 |
| Carbocernaite | 3.00 / 3.00 | 3.53 |
| Cebaite-(Ce) | 4.50 / 5.00 | 4.81 |
| Cebaite-(Nd) | 4.50 / 5.00 | 4.80 |
| Celsian | 6.00 / 6.50 | 3.10 |
| Cerchiaraite-(Al) | 4.50 / 4.50 | 3.64 |
| Cerchiaraite-(Fe) | 4.50 / 4.50 | 3.71 |
| Cerchiaraite-(Mn) | 4.00 / 5.00 | |
| Charoite | 5.00 / 6.00 | 2.54 |
| Chernykhite | 3.00 / 4.00 | 3.14 |
| Chromphyllite | 3.00 / 3.00 | 2.88 |
| Clinobarylite | 6.50 / 6.50 | 3.97 |
| Clinoptilolite-Na | 3.50 / 4.00 | 2.10 |
| Colinowensite | ||
| Cordylite-(Ce) | 4.50 / 4.50 | 4.44 |
| Cordylite-(La) | 4.00 / 4.00 | 4.33 |
| Coronadite | 4.50 / 5.00 | 5.44 |
| Coutinhoite | 1.50 / 1.50 | |
| Curetonite | 3.50 / 3.50 | 4.31 |
| Cymrite | 2.00 / 3.00 | 3.41 |
| Dachiardite-Ca | 4.00 / 4.50 | 2.14 |
| Dachiardite-Na | 4.00 / 5.00 | 2.16 |
| Daqingshanite-(Ce) | 5.00 / 5.00 | 3.81 |
| Dargaite | ||
| Delindeite | 3.30 | |
| Devitoite | 4.00 / 4.00 | 4.04 |
| Dickinsonite-(KMnNa) | 3.50 / 4.00 | 3.42 |
| Direnzoite | 4.50 / 4.50 | 2.12 |
| Diversilite-(Ce) | 5.00 / 5.00 | 3.68 |
| Dresserite | 2.50 / 3.00 | 2.96 |
| Dualite | 5.00 / 5.00 | 2.84 |
| Dussertite | 3.50 / 3.50 | 3.75 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





