List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
In - Indium - POOR METAL
Indium is a metallic chemical element belonging to group 13 and period 5 of the periodic table of elements. With atomic symbol In and atomic number 49, indium has 13 known isotopes, with an atomic mass varying between 121 and 133. It has a relative atomic weight of 114.82.
indium has a melting point of 156.60°C and a boiling point of 2072°C. It has a relative density of 7.29 g/cm3 and an oxidation state of +3. Its color is greyish-white when pure, but it takes on a reddish color when heated.
indium is primarily used to create alloys for industrial uses. It can be added to steel to make it more corrosion-resistant. It is also used to produce alloys with tin, which is useful in electronics and electronic devices. In addition, indium also plays an important role in the manufacture of electronic components.
Besides these industrial uses, indium is also used to produce refractory materials for thermal insulation. It is also used in solar cells and sensors. Finally, it is used to produce non-stick coatings for kitchen utensils.
indium has a melting point of 156.60°C and a boiling point of 2072°C. It has a relative density of 7.29 g/cm3 and an oxidation state of +3. Its color is greyish-white when pure, but it takes on a reddish color when heated.
indium is primarily used to create alloys for industrial uses. It can be added to steel to make it more corrosion-resistant. It is also used to produce alloys with tin, which is useful in electronics and electronic devices. In addition, indium also plays an important role in the manufacture of electronic components.
Besides these industrial uses, indium is also used to produce refractory materials for thermal insulation. It is also used in solar cells and sensors. Finally, it is used to produce non-stick coatings for kitchen utensils.
Synthetic
Radioactive
Liquid
Gaseous
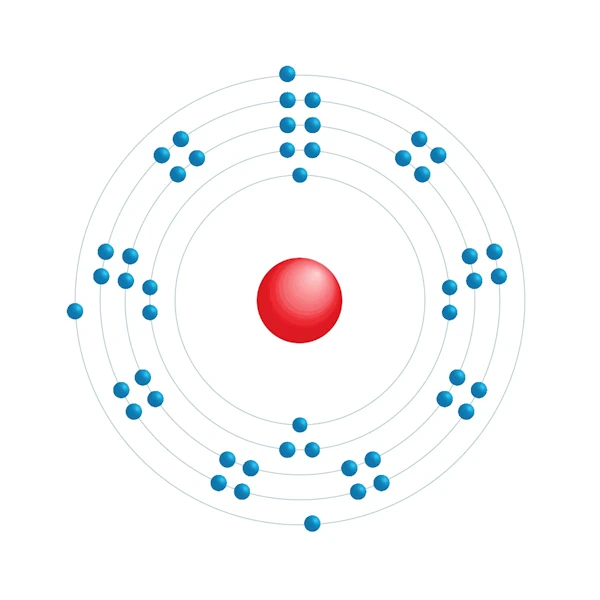
Electronic configuration diagram
| Name | Indium |
| Number | 49 |
| Atomic | 114.818 |
| Symbol | In |
| Fusion | 156.2 |
| Boiling | 2080 |
| Density | 7.31 |
| Period | 5 |
| Group | 13 |
| Discovery | 1863 Reich and Richter |
| Abundance | 0.25 |
| Radius | 2 |
| Electronegativity | 1.78 |
| Ionization | 5.7864 |
| Number of isotopes | 34 |
| Electronic configuration | [Kr] 4d10 5s2 5p1 |
| Oxidation states | 1,2,3 |
| Electron by energy level | 2,8,18,18,3 |
| Mineral | Hardness | Density |
| Abramovite | ||
| Cadmoindite | ||
| Damiaoite | 5.00 / 5.00 | 10.95 |
| Dzhalindite | 4.00 / 4.50 | 4.34 |
| Indite | 5.00 / 5.00 | 4.67 |
| Indium | 3.50 / 3.50 | 7.20 |
| Ishiharaite | ||
| Kudriavite | 6.58 | |
| Laforêtite | 3.00 / 3.00 | 4.93 |
| Petrukite | 4.50 / 4.50 | 4.61 |
| Roquesite | 3.50 / 4.00 | 4.00 |
| Sakuraiite | 4.00 / 4.00 | 4.00 |
| Yanomamite | 5.50 / 6.00 | 3.87 |
| Yixunite | 6.00 / 6.00 | 18.21 |
| Znamenskyite |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





