List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
Cs - cesium - ALKALI METAL
Cesium is a group 1 metal of the periodic table. Its chemical element is Cs and it has an atomic number of 55. Its atomic mass is 132.905 g/mol and its melting point is 28°C, which makes it very malleable and gives it a very low density. It has a silver color and glows in the dark, earning it the nickname "shiny metal".
cesium has very high reactive properties. It reacts very easily with oxygen and water and can even react with copper at room temperature. It is very widely used in industry as a catalyst in chemical processes, especially during the production of petroleum products.
From a medical point of view, cesium is used to treat various diseases, including cancer and Parkinson's disease. It is also used as a base chemical in the manufacture of drugs and pharmaceutical compounds.
cesium is also used in the electronics industry because of its conductive properties and its ability to produce alpha particles. It is widely used in the manufacture of diodes, transistors and integrated circuits.
cesium is also used in halogen lamps and works of art, where it is used for its exceptional thermal and galvanic properties. Metallurgy and the aerospace industry also use cesium for its mechanical and electrochemical properties.
cesium has very high reactive properties. It reacts very easily with oxygen and water and can even react with copper at room temperature. It is very widely used in industry as a catalyst in chemical processes, especially during the production of petroleum products.
From a medical point of view, cesium is used to treat various diseases, including cancer and Parkinson's disease. It is also used as a base chemical in the manufacture of drugs and pharmaceutical compounds.
cesium is also used in the electronics industry because of its conductive properties and its ability to produce alpha particles. It is widely used in the manufacture of diodes, transistors and integrated circuits.
cesium is also used in halogen lamps and works of art, where it is used for its exceptional thermal and galvanic properties. Metallurgy and the aerospace industry also use cesium for its mechanical and electrochemical properties.
Synthetic
Radioactive
Liquid
Gaseous
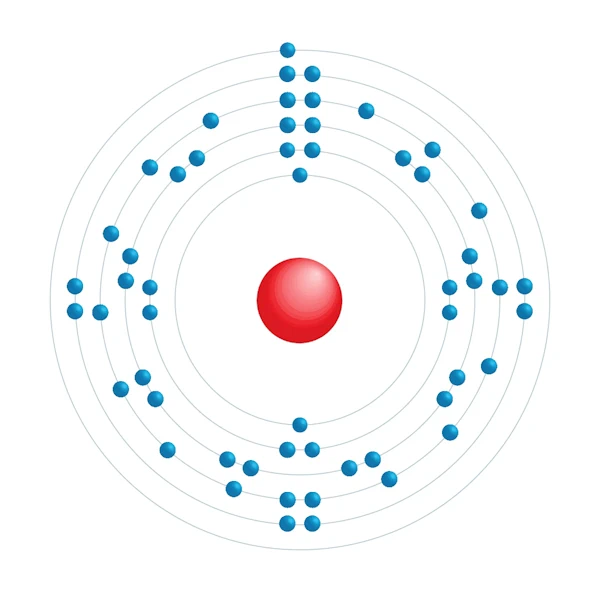
Electronic configuration diagram
| Name | cesium |
| Number | 55 |
| Atomic | 132.90545196 |
| Symbol | Cs |
| Fusion | 28.4 |
| Boiling | 690 |
| Density | 1.873 |
| Period | 6 |
| Group | 1 |
| Discovery | 1860 Bunsen and Kirchoff |
| Abundance | 3 |
| Radius | 3.3 |
| Electronegativity | 0.79 |
| Ionization | 3.8939 |
| Number of isotopes | 22 |
| Electronic configuration | [Xe] 6s1 |
| Oxidation states | 1 |
| Electron by energy level | 2,8,18,18,8,1 |
| Mineral | Hardness | Density |
| Avogadrite | 2.62 | |
| Caesiumpharmacosiderite | ||
| Cesiodymite | ||
| Cesplumtantite | 7.00 / 7.00 | 6.00 |
| Dachiardite-Ca | 4.00 / 4.50 | 2.14 |
| Gainesite | 4.00 / 4.00 | 2.94 |
| Galkhaite | 3.00 / 3.00 | 5.40 |
| Kirchhoffite | 6.00 / 6.50 | 3.64 |
| Kupletskite-(Cs) | 4.00 / 4.00 | 3.68 |
| Londonite | 8.00 / 8.00 | 3.34 |
| Margaritasite | 2.00 / 2.00 | 5.40 |
| Mccrillisite | 4.50 / 4.50 | 3.12 |
| Mendeleevite-(Ce) | ||
| Mendeleevite-(Nd) | 5.00 / 5.50 | 3.16 |
| Nalivkinite | 3.29 | |
| Nanpingite | 2.50 / 3.00 | 3.11 |
| Natrobistantite | 6.10 | |
| Odigitriaite | 5.00 / 5.00 | 2.83 |
| Pautovite | 2.50 / 2.50 | 3.85 |
| Pezzottaite | 8.00 / 8.00 | 2.97 |
| Pollucite | 6.50 / 6.50 | 2.90 |
| Potassicmendeleevite-(Ce) | ||
| Ramanite-(Cs) | ||
| Rhodizite | 8.50 / 8.50 | 3.44 |
| Senkevichite | 5.50 / 6.00 | 3.13 |
| Sokolovaite | ||
| Telyushenkoite | 6.00 / 6.00 | 2.73 |
| Zeravshanite | 6.00 / 6.00 | 3.09 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





