List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Tc technetium
Tc - technetium - TRANSITION METAL
Technetium (Tc) is a metal belonging to period 5 of the periodic table and to the vanadium group family. It is the first radioactive element to have been synthesized and it is also the lightest of the known radioactive elements. technetium is the only radioactive element that is not found in its native state, but only in the form of radioactive isotopes. It is present in the environment in small amounts as a result of natural radioactive activity.
technetium has an average atomic mass of 98.91 u and has an atomic number of 43. Depending on its natural state, technetium can be obtained in the form of its metallic form or in the form of its radioactive isotopes. Due to its radioactivity, technetium metal is unstable and breaks down into a variety of oxygen-like products.
technetium is mainly used in the medical field, especially in the diagnosis and treatment of heart disease and certain forms of cancer. It is also used in the production of nuclear weapons and refractory materials and to make labeled radioactive isotopes to treat autoimmune diseases and to identify chemical compounds. In addition, technetium is used in research to compare the effectiveness of drugs.
technetium has an average atomic mass of 98.91 u and has an atomic number of 43. Depending on its natural state, technetium can be obtained in the form of its metallic form or in the form of its radioactive isotopes. Due to its radioactivity, technetium metal is unstable and breaks down into a variety of oxygen-like products.
technetium is mainly used in the medical field, especially in the diagnosis and treatment of heart disease and certain forms of cancer. It is also used in the production of nuclear weapons and refractory materials and to make labeled radioactive isotopes to treat autoimmune diseases and to identify chemical compounds. In addition, technetium is used in research to compare the effectiveness of drugs.
Synthetic
Radioactive
Liquid
Gaseous
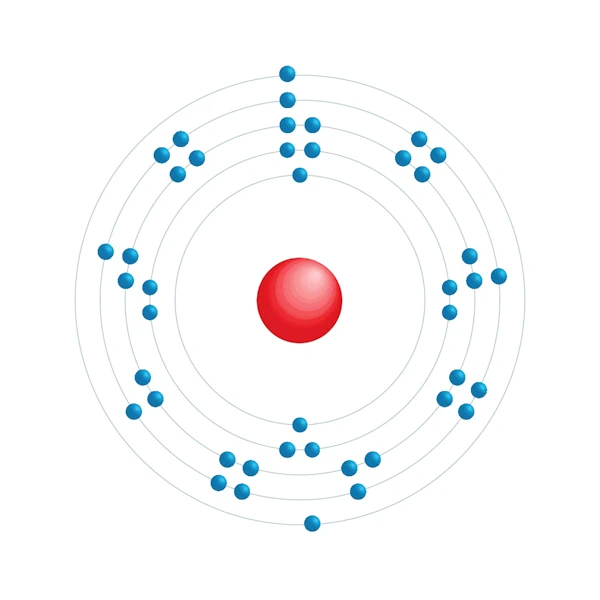
Electronic configuration diagram
| Name | technetium |
| Number | 43 |
| Atomic | 98 |
| Symbol | Tc |
| Fusion | 2172 |
| Boiling | 5030 |
| Density | 11.5 |
| Period | 5 |
| Group | 7 |
| Discovery | 1937 Perrier and Segre |
| Abundance | 0.001 |
| Radius | 2 |
| Electronegativity | 1.9 |
| Ionization | 7.28 |
| Number of isotopes | 23 |
| Electronic configuration | [Kr] 4d5 5s2 |
| Oxidation states | -3,-1,1,2,3,4,5,6,7 |
| Electron by energy level | 2,8,18,13,2 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





