List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
Ar - Argon - NO NOBLE GAS METAL
Argon is a chemical element of the family of noble gases, symbol Ar and atomic number 18. It is produced by fractional separation of gases present in the air, mainly by oxygen and nitrogen. It is odorless, colorless and inert.
argon is an inert gas in gaseous state, which forms eight-atom molecules (Argon Atoms – Ar8). Its molar mass is 39.948 g/mol. Its characteristics make it very stable in aseptic environments. It is the third most abundant noble gas in the atmosphere, making up 0.94% of the air. Its boiling temperature and vapor pressure saturate at –186°C and 0.52 MPa respectively.
argon is a poor thermal and electrical conductor, and is therefore used for soldering and filling electronic products and for filling fluorescent lamps. It is also used as a shielding gas, as it is non-combustible and will not react with other molecules.
argon is used in many applications, including metallurgy, for filling fires, for manufacturing chemicals and insulation, for filling fluorescent lamps, and for inerting pressurized vessels and enclosures where high temperatures are used. argon is also used in gas chromatography analysis to separate mixtures and measure the concentration of individual gases. Finally, argon is used for the manufacture of drugs, for example the painkiller lidocaine.
argon is an inert gas in gaseous state, which forms eight-atom molecules (Argon Atoms – Ar8). Its molar mass is 39.948 g/mol. Its characteristics make it very stable in aseptic environments. It is the third most abundant noble gas in the atmosphere, making up 0.94% of the air. Its boiling temperature and vapor pressure saturate at –186°C and 0.52 MPa respectively.
argon is a poor thermal and electrical conductor, and is therefore used for soldering and filling electronic products and for filling fluorescent lamps. It is also used as a shielding gas, as it is non-combustible and will not react with other molecules.
argon is used in many applications, including metallurgy, for filling fires, for manufacturing chemicals and insulation, for filling fluorescent lamps, and for inerting pressurized vessels and enclosures where high temperatures are used. argon is also used in gas chromatography analysis to separate mixtures and measure the concentration of individual gases. Finally, argon is used for the manufacture of drugs, for example the painkiller lidocaine.
Synthetic
Radioactive
Liquid
Gaseous
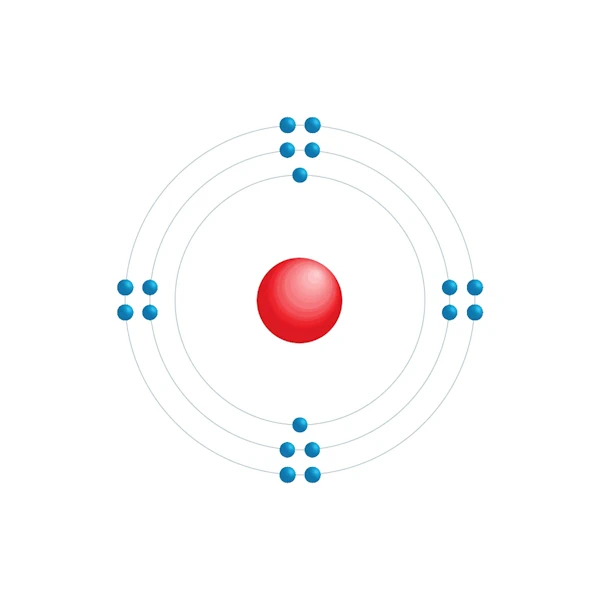
Electronic configuration diagram
| Name | Argon |
| Number | 18 |
| Atomic | 39.948 |
| Symbol | Ar |
| Fusion | -189.4 |
| Boiling | -185.9 |
| Density | 0.0017837 |
| Period | 3 |
| Group | 18 |
| Discovery | 1894 Rayleigh and Ramsay |
| Abundance | 3.5 |
| Radius | 0.88 |
| Electronegativity | 0 |
| Ionization | 15.7596 |
| Number of isotopes | 8 |
| Electronic configuration | [Ne] 3s2 3p6 |
| Oxidation states | 0 |
| Electron by energy level | 2,8,8 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





