List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Tl Thallium
Tl - Thallium - POOR METAL
Synthetic
Radioactive
Liquid
Gaseous
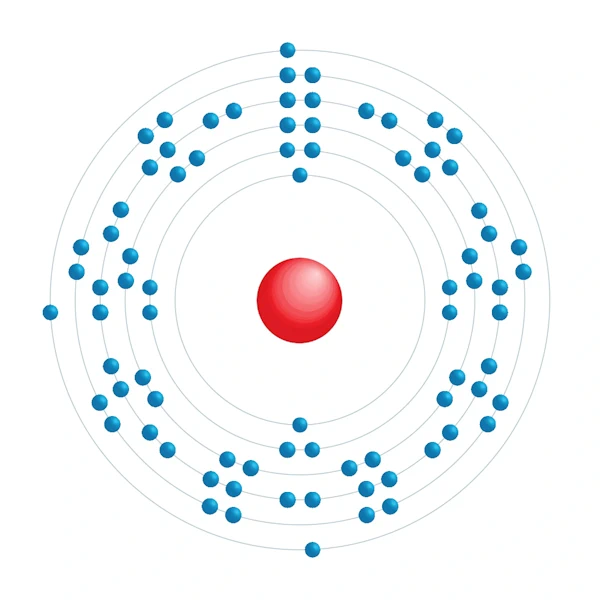
Electronic configuration diagram
| Name | Thallium |
| Number | 81 |
| Atomic | 204.389 |
| Symbol | Tl |
| Fusion | 303.6 |
| Boiling | 1457 |
| Density | 11.85 |
| Period | 6 |
| Group | 13 |
| Discovery | 1861 Crookes |
| Abundance | 0.85 |
| Radius | 2.1 |
| Electronegativity | 2.04 |
| Ionization | 6.1082 |
| Number of isotopes | 28 |
| Electronic configuration | [Xe] 4f14 5d10 6s2 6p1 |
| Oxidation states | 1,3 |
| Electron by energy level | 2,8,18,32,18,3 |
| Mineral | Hardness | Density |
| Arsiccioite | 2.00 / 2.50 | 5.99 |
| Avicennite | 2.00 / 2.00 | 9.57 |
| Bernardite | 2.00 / 2.00 | 4.50 |
| Boscardinite | 3.00 | |
| Bukovite | 2.00 / 2.00 | 7.36 |
| Carlinite | 1.00 / 1.00 | 8.10 |
| Chabournéite | 3.00 / 3.50 | 5.10 |
| Chalcothallite | 2.00 / 2.50 | 6.60 |
| Christite | 1.00 / 2.00 | 6.20 |
| Chrysothallite | 2.97 | |
| Criddleite | 3.00 / 3.50 | 6.00 |
| Crookesite | 2.50 / 3.00 | 6.90 |
| Cuprostibite | 4.00 / 4.00 | 8.42 |
| Dalnegroite | 3.00 / 3.50 | |
| Dorallcharite | 3.00 / 4.00 | 3.85 |
| Ecrinsite | ||
| Edenharterite | 2.50 / 3.00 | 5.00 |
| Ellisite | 2.00 / 2.00 | 7.10 |
| Enneasartorite | ||
| Erniggliite | 2.00 / 3.00 | 5.00 |
| Evdokimovite | ||
| Fangite | 2.00 / 2.50 | 6.19 |
| Ferrostalderite | ||
| Fettelite | 3.50 / 4.00 | 6.29 |
| Gabrielite | 1.50 / 2.00 | 5.38 |
| Galkhaite | 3.00 / 3.00 | 5.40 |
| Gillulyite | 2.00 / 2.50 | 4.02 |
| Hatchite | 5.00 | |
| Hendekasartorite | ||
| Hephaistosite | ||
| Heptasartorite | ||
| Honeaite | 11.18 | |
| Hutchinsonite | 1.50 / 2.00 | 4.60 |
| Imhofite | 2.00 / 2.00 | 4.39 |
| Jankovicite | 2.00 / 2.00 | 5.08 |
| Jentschite | 2.00 / 2.50 | 5.24 |
| Karpovite | 2.00 / 2.00 | 4.74 |
| Lafossaite | 3.00 / 4.00 | 7.21 |
| Lanmuchangite | 3.00 / 3.50 | 2.22 |
| Lorándite | 2.00 / 2.50 | 5.53 |
| Markhininite | 5.91 | |
| Nataliyamalikite | ||
| Parapierrotite | 2.50 / 3.00 | 5.04 |
| Pautovite | 2.50 / 2.50 | 3.85 |
| Perlialite | 4.00 / 5.00 | 2.14 |
| Philrothite | 3.00 / 3.50 | |
| Picotpaulite | 2.00 / 2.00 | 5.20 |
| Pierrotite | 3.50 / 3.50 | 4.97 |
| Protochabournéite | ||
| Raberite | 2.50 / 3.00 | |
| Raguinite | 6.40 | |
| Ralphcannonite | ||
| Rathite | 3.00 / 3.00 | 5.41 |
| Rayite | 2.50 / 2.50 | 6.00 |
| Rebulite | 4.81 | |
| Rohaite | 3.00 / 3.50 | 7.78 |
| Routhierite | 3.50 / 3.50 | 5.83 |
| Sabatierite | 2.50 / 2.50 | 6.00 |
| Sartorite | 3.00 / 3.00 | 5.10 |
| Sicherite | 3.00 / 3.00 | |
| Simonite | ||
| Spaltiite | ||
| Stalderite | 3.50 / 4.00 | 4.97 |
| Steropesite | 5.74 | |
| Thalcusite | 2.50 / 3.00 | 6.15 |
| Thalfenisite | 1.00 / 1.50 | 5.26 |
| Thalliumpharmacosiderite | ||
| Vaughanite | 3.00 / 3.50 | 5.00 |
| Vrbaite | 3.50 / 3.50 | 5.30 |
| Vurroite | 6.15 | |
| Wallisite | 5.00 | |
| Weissbergite | 1.50 / 1.50 | 5.79 |
| Wilhelmramsayite | 2.50 / 2.50 | 2.75 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





