List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Cr Chromium
Cr - Chromium - TRANSITION METAL
Chromium is a chemical element that is found in the metal group of the periodic table of elements, symbolized by the symbol Cr and having an atomic number of 24. It is a metallic element that has interesting properties.
chromium is a hard but relatively flexible metal. It has good ductility and is very resistant to corrosion. It has a metallic white color and a characteristic gloss.
chromium is a ductile and malleable metal that is often used in alloys with other metals to give them corrosion resistance and durability. It is also used for its ability to produce brilliant color and heat resistant coatings.
chromium is widely used for its resistance to corrosion and abrasion. It is commonly used for making pipes, tanks, refrigerators, stoves, ovens and vehicles. It is also used to make automotive components, medical instruments and electronic devices. Chromate complexes are also used as colorants and anti-corrosives for industrial coatings.
chromium is a hard but relatively flexible metal. It has good ductility and is very resistant to corrosion. It has a metallic white color and a characteristic gloss.
chromium is a ductile and malleable metal that is often used in alloys with other metals to give them corrosion resistance and durability. It is also used for its ability to produce brilliant color and heat resistant coatings.
chromium is widely used for its resistance to corrosion and abrasion. It is commonly used for making pipes, tanks, refrigerators, stoves, ovens and vehicles. It is also used to make automotive components, medical instruments and electronic devices. Chromate complexes are also used as colorants and anti-corrosives for industrial coatings.
Synthetic
Radioactive
Liquid
Gaseous
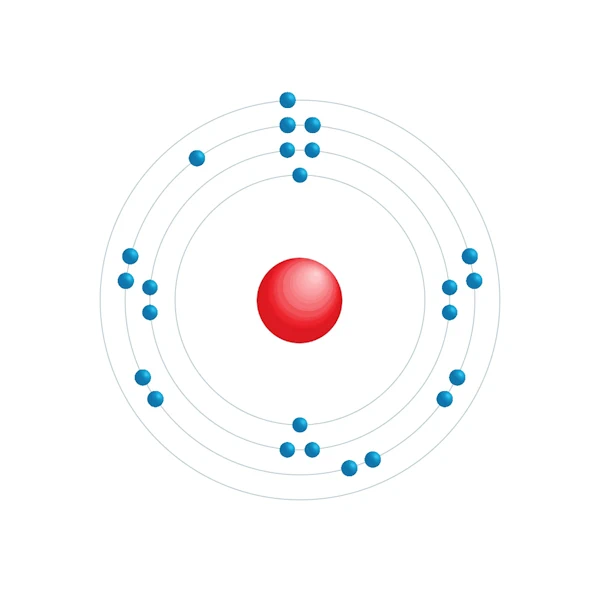
Electronic configuration diagram
| Name | Chromium |
| Number | 24 |
| Atomic | 51.9961 |
| Symbol | Cr |
| Fusion | 1857 |
| Boiling | 2482 |
| Density | 7.15 |
| Period | 4 |
| Group | 6 |
| Discovery | 1797 Vauquelin |
| Abundance | 102 |
| Radius | 1.9 |
| Electronegativity | 1.66 |
| Ionization | 6.7665 |
| Number of isotopes | 9 |
| Electronic configuration | [Ar] 3d5 4s1 |
| Oxidation states | -2,-1,1,2,3,4,5,6 |
| Electron by energy level | 2,8,13,1 |
| Mineral | Hardness | Density |
| Andreyivanovite | ||
| Ankangite | 6.50 / 6.50 | 4.44 |
| Ardennite-(V) | 6.00 / 7.00 | 3.55 |
| Barbertonite | 1.50 / 2.00 | 2.10 |
| Batisivite | 7.00 / 7.00 | 4.62 |
| Bentorite | 2.00 / 2.00 | 2.03 |
| Bouazzerite | 2.81 | |
| Bracewellite | 5.50 / 6.50 | 4.45 |
| Brezinaite | 3.50 / 4.50 | 4.12 |
| Bushmakinite | 3.00 / 3.50 | 6.22 |
| Carlosruizite | 2.50 / 3.00 | 3.42 |
| Carlsbergite | 7.00 / 7.00 | 5.90 |
| Carmichaelite | 6.00 / 6.00 | 4.13 |
| Cassedanneite | 3.50 / 3.50 | 6.00 |
| Caswellsilverite | 1.00 / 2.00 | 3.21 |
| Chromatite | 3.14 | |
| Chrombismite | 3.00 / 3.50 | 9.80 |
| Chromceladonite | 1.00 / 2.00 | 2.90 |
| Chromferide | 4.00 / 4.00 | 6.69 |
| Chromio-pargasite | 6.00 / 6.00 | 3.12 |
| Chromite | 5.50 / 5.50 | 4.50 |
| Chromium | 7.50 / 7.50 | 7.20 |
| Chromium diopside | 5.50 / 6.50 | 3.30 |
| Chromium-dravite | 7.00 / 7.50 | |
| Chromo-alumino-povondraite | 7.50 / 7.50 | 3.23 |
| Chromomphacite | ||
| Chromphyllite | 3.00 / 3.00 | 2.88 |
| Chromschieffelinite | 2.00 / 2.00 | 5.89 |
| Cochromite | 7.00 / 7.00 | 5.22 |
| Crichtonite | 5.00 / 6.00 | 4.46 |
| Crocoite | 2.50 / 3.00 | 5.90 |
| Cronusite | 1.50 / 1.50 | 2.51 |
| Cuprokalininite | 4.50 / 5.00 | 4.16 |
| Daubréelite | 4.50 / 5.00 | 3.81 |
| Davidite-(Ce) | 6.00 / 6.00 | 4.42 |
| Davidite-(La) | 6.00 / 6.00 | 4.42 |
| Deanesmithite | 4.50 / 5.00 | 8.00 |
| Dessauite-(Y) | 6.50 / 7.00 | 4.68 |
| Dietzeite | 3.50 / 3.50 | 3.62 |
| Dissakisite-(La) | 6.50 / 7.00 | 3.79 |
| Dukeite | 3.00 / 4.00 | 7.17 |
| Edoylerite | 7.00 | |
| Embreyite | 3.50 / 3.50 | 6.45 |
| Eskolaite | 8.00 / 8.50 | 5.18 |
| Ferchromide | 6.50 / 6.50 | 6.00 |
| Ferrohögbomite-2N2S | 6.00 / 7.00 | |
| Florensovite | 5.00 / 5.00 | 4.00 |
| Fornacite | 2.00 / 3.00 | 6.27 |
| George-ericksenite | 3.00 / 4.00 | 3.04 |
| Georgerobinsonite | ||
| Grimaldiite | 3.50 / 4.50 | 4.11 |
| Guyanaite | 4.53 | |
| Hapkeite | ||
| Hashemite | 3.50 / 3.50 | 4.59 |
| Hawthorneite | 6.00 / 6.50 | 5.00 |
| Heideite | 3.50 / 4.50 | 4.10 |
| Hemihedrite | 3.00 / 3.00 | 6.42 |
| Iquiqueite | 2.00 / 2.00 | 2.05 |
| Iranite | 3.00 / 3.00 | 5.80 |
| Isovite | 8.00 / 8.00 | 7.40 |
| Jade | 6.00 / 6.50 | 3.00 |
| Joegoldsteinite | 3.71 | |
| Kalininite | 5.00 / 5.00 | |
| Kassite | 5.00 / 5.00 | 3.42 |
| Keilite | ||
| Khristovite-(Ce) | 5.00 / 5.00 | 4.05 |
| Knorringite | 6.00 / 7.00 | 3.76 |
| Kosmochlor | 6.00 / 7.00 | 3.60 |
| Krinovite | 6.00 / 7.00 | 3.38 |
| Lakargiite | 8.00 / 8.50 | 4.59 |
| Lindsleyite | 7.50 / 7.50 | 4.63 |
| Lópezite | 2.50 / 2.50 | 2.69 |
| Loveringite | 7.50 / 7.50 | 4.41 |
| Macquartite | 3.50 / 3.50 | 5.49 |
| Magnesiochromite | 5.50 / 5.50 | 4.20 |
| Magnesiosadanagaite | 5.50 / 6.00 | 3.00 |
| Manganochromite | 5.50 / 5.50 | 4.86 |
| Mapiquiroite | 6.00 / 6.00 | |
| Mariinskite | 8.50 / 8.50 | 4.25 |
| Mathiasite | 7.50 / 7.50 | 4.60 |
| Mcconnellite | 5.50 / 5.50 | 5.49 |
| Molybdofornacite | 2.00 / 3.00 | 6.60 |
| Mongshanite | 5.00 / 6.00 | |
| Mountkeithite | 2.00 / 2.00 | 2.12 |
| Murchisite | 4.22 | |
| Natalyite | 7.00 / 7.00 | 3.55 |
| Nichromite | 6.00 / 6.50 | 5.24 |
| Olkhonskite | 8.00 / 8.00 | 4.48 |
| Oxy-chromium-dravite | 7.50 / 7.50 | 3.30 |
| Painite | 8.00 / 8.00 | 4.00 |
| Petterdite | 2.00 / 2.00 | 3.97 |
| Phoenicochroite | 2.50 / 2.50 | 7.01 |
| Polyakovite-(Ce) | 5.50 / 6.00 | 4.75 |
| Putnisite | 1.00 / 2.00 | 2.23 |
| Qusongite | 9.50 / 9.50 | 15.84 |
| Redingtonite | 2.00 / 2.00 | 1.76 |
| Redledgeite | 6.00 / 7.00 | 3.72 |
| Reynoldsite | 4.50 / 4.50 | 6.57 |
| Rilandite | 2.00 / 3.00 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





