List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Ca Calcium
Ca - Calcium - ALKALINE EARTH METAL
Calcium (chemical symbol Ca) is a metallic chemical element that occurs as a silver-gray metal in the free state and as tricalcium phosphate or calcium carbonate in the form of salts. This chemical element is the fifth most abundant element on earth and the third in the oceans. It is one of the alkali metals.
calcium is a silvery gray metal in its pure state. Its molar mass is 40.08 g/mol and its atomic number is 20. It has a melting point of 1442°C and a boiling point of 2572°C. Its density is 1.55 g/cm3. Its crystal structure is face-centered cubic and its valence is 2+.
calcium is a reactive metal that easily combines with oxygens and halogens to form salts, as well as acids to form organic acid salts. It is very soluble in water and is insoluble in organic solvents. It is a good catalyst and is capable of fixing oxygen or hydroxyl ions.
calcium is widely used in the cement, facade coatings and adhesives industry. It is also used in the manufacture of dairy products, matches, food coloring and building materials. In medicine, calcium is used as a food additive to increase the absorption of iron and phosphorus. CaCO3 is used to adsorb carbon dioxide and regulate the pH of swimming pool water. Finally, calcium is an important component of the skeleton in humans and animals.
calcium is a silvery gray metal in its pure state. Its molar mass is 40.08 g/mol and its atomic number is 20. It has a melting point of 1442°C and a boiling point of 2572°C. Its density is 1.55 g/cm3. Its crystal structure is face-centered cubic and its valence is 2+.
calcium is a reactive metal that easily combines with oxygens and halogens to form salts, as well as acids to form organic acid salts. It is very soluble in water and is insoluble in organic solvents. It is a good catalyst and is capable of fixing oxygen or hydroxyl ions.
calcium is widely used in the cement, facade coatings and adhesives industry. It is also used in the manufacture of dairy products, matches, food coloring and building materials. In medicine, calcium is used as a food additive to increase the absorption of iron and phosphorus. CaCO3 is used to adsorb carbon dioxide and regulate the pH of swimming pool water. Finally, calcium is an important component of the skeleton in humans and animals.
Synthetic
Radioactive
Liquid
Gaseous
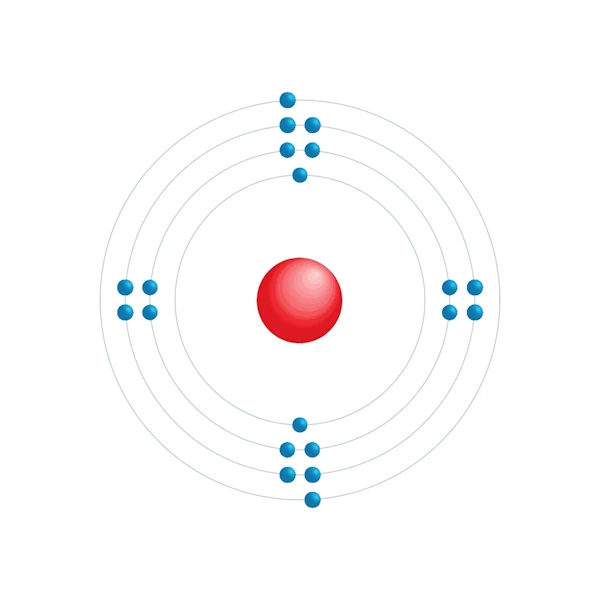
Electronic configuration diagram
| Name | Calcium |
| Number | 20 |
| Atomic | 40.078 |
| Symbol | Ca |
| Fusion | 839 |
| Boiling | 1487 |
| Density | 1.54 |
| Period | 4 |
| Group | 2 |
| Discovery | 1808 Davy |
| Abundance | 41500 |
| Radius | 2.2 |
| Electronegativity | 1 |
| Ionization | 6.1132 |
| Number of isotopes | 14 |
| Electronic configuration | [Ar] 4s2 |
| Oxidation states | 2 |
| Electron by energy level | 2,8,8,2 |
| Mineral | Hardness | Density |
| Abuite | ||
| Actinolite | 5.50 / 5.50 | 2.98 |
| Adachiite | ||
| Addibischoffite | ||
| Adelite | 5.00 / 5.00 | 3.73 |
| Adrianite | ||
| Aegirine-augite | 6.00 / 6.00 | 3.40 |
| Aenigmatite | 5.00 / 6.00 | 3.74 |
| Aerinite | 3.00 / 3.00 | 2.48 |
| Aeschynite-(Ce) | 5.00 / 6.00 | 5.19 |
| Aeschynite-(Y) | 5.00 / 6.00 | 4.85 |
| Afghanite | 5.50 / 6.00 | 2.55 |
| Afwillite | 3.00 / 3.00 | 2.62 |
| Agakhanovite-(Y) | 6.00 / 6.00 | 2.67 |
| Agardite-(Ce) | 3.00 / 3.00 | 3.72 |
| Agardite-(La) | 3.00 / 4.00 | 3.72 |
| Agardite-(Nd) | 3.00 / 4.00 | 3.72 |
| Agardite-(Y) | 3.00 / 4.00 | 3.66 |
| Agrellite | 5.50 / 5.50 | 2.88 |
| Agrinierite | 5.62 | |
| Akatoreite | 6.00 / 6.00 | 3.48 |
| Akermanite | 5.00 / 6.00 | 2.94 |
| Aklimaite | ||
| Albite | 7.00 / 7.00 | 2.61 |
| Albrechtschraufite | 2.00 / 3.00 | 2.60 |
| Aldridgeite | 3.00 / 3.00 | 3.33 |
| Aleksandrovite | 4.00 / 4.50 | 3.07 |
| Alexkhomyakovite | ||
| Alflarsenite | 4.00 / 4.00 | 2.61 |
| Alfredstelznerite | ||
| Aliettite | 1.00 / 2.00 | |
| Allanite-(Ce) | 5.50 / 5.50 | 3.30 |
| Allanite-(La) | 6.00 / 6.00 | 3.93 |
| Allanite-(Nd) | ||
| Allanite-(Y) | 5.50 / 5.50 | 3.30 |
| Allendeite | 4.84 | |
| Alloriite | 5.00 / 5.00 | 2.35 |
| Alluaivite | 5.00 / 6.00 | 2.76 |
| Alluaudite | 5.00 / 5.50 | 3.45 |
| Almarudite | 6.00 / 6.00 | 2.71 |
| Alnaperbøeite-(Ce) | ||
| Alsakharovite-Zn | 5.00 / 5.00 | 2.90 |
| Alstonite | 4.00 / 4.50 | 3.69 |
| Alumino-ferrobarroisite | ||
| Alumino-ferrohornblende | ||
| Alumino-ferrotschermakite | ||
| Alumino-ferrowinchite | ||
| Alumino-magnesiohornblende | ||
| Alumino-magnesiotaramite | ||
| Aluminobarroisite | 5.00 / 6.00 | 2.94 |
| Aluminocerite-(Ce) | 5.00 / 5.00 | 4.68 |
| Aluminokatophorite | 5.00 / 6.00 | |
| Aluminotschermakite | 5.00 / 6.00 | |
| Aluminowinchite | ||
| Alumoåkermanite | 4.00 / 5.00 | 3.00 |
| Alumohydrocalcite | 2.50 / 2.50 | 2.23 |
| Alumotungstite | ||
| Alumovesuvianite | ||
| Aminoffite | 5.50 / 5.50 | 2.94 |
| Ammonite | 6.00 / 7.00 | 3.20 |
| Amstallite | 4.00 / 4.00 | 2.40 |
| Anapaite | 3.00 / 4.00 | 2.80 |
| Andersonite | 2.50 / 2.50 | 2.79 |
| Andesine | 7.00 / 7.00 | 2.66 |
| Andradite | 6.50 / 7.00 | 3.70 |
| Andrianovite | 5.00 / 5.00 | 3.02 |
| Angastonite | 2.00 / 2.00 | 2.47 |
| Angelite | 3.50 / 3.50 | 2.96 |
| Anhydrite | 3.50 / 3.50 | 2.96 |
| Ankerite | 3.50 / 4.00 | 3.00 |
| Anorthite | 6.00 / 6.00 | 2.72 |
| Antarcticite | 2.00 / 3.00 | 1.71 |
| Apatite | 5.00 / 6.00 | 3.16 |
| Aqualite | 4.00 / 5.00 | 2.66 |
| Aradite | ||
| Aragonite | 3.50 / 4.00 | 2.93 |
| Arapovite | 5.50 / 6.00 | 3.43 |
| Arctite | 5.00 / 5.00 | 3.11 |
| Ardealite | 1.00 / 1.50 | 2.30 |
| Ardennite-(As) | 6.00 / 7.00 | 3.62 |
| Ardennite-(V) | 6.00 / 7.00 | 3.55 |
| Armbrusterite | 3.50 / 3.50 | 2.78 |
| Armenite | 7.50 / 7.50 | 2.76 |
| Armstrongite | 4.50 / 4.50 | 2.70 |
| Arnhemite | 2.33 | |
| Arrojadite-(BaFe) | 3.54 | |
| Arrojadite-(BaNa) | ||
| Arrojadite-(KFe) | 5.00 / 5.00 | 3.50 |
| Arrojadite-(KNa) | ||
| Arrojadite-(NaFe) | ||
| Arrojadite-(PbFe) | 4.00 / 5.00 | |
| Arrojadite-(SrFe) | ||
| Arseniopleite | 3.50 / 3.50 | 4.22 |
| Arseniosiderite | 1.50 / 1.50 | 3.50 |
| Arsenocrandallite | 5.50 / 5.50 | 3.25 |
| Arsenogoyazite | 4.00 / 4.00 | 3.33 |
| Arsenuranylite | 2.50 / 2.50 | 4.25 |
| Asbecasite | 6.50 / 7.00 | 3.70 |
| Asbolane | 6.00 / 6.00 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





