List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Rb Rubidium
Rb - Rubidium - ALKALI METAL
Rubidium is a metallic chemical element that is found in group 1 (alkaline) of the periodic table. Its chemical symbol is Rb and its atomic number is 37. Its name comes from the Latin word "rubidius" which means "red", due to the bright red color rubidium vapor produces when exposed to air.
rubidium is the most reactive chemical element of the alkalis, and it has an external electronic configuration similar to that of potassium and sodium. It is also the most abundant of the alkalines.
rubidium metal is very malleable and ductile and is more volatile than potassium and sodium. It is soluble in water and turns into Rb+ ions. This ion is strongly oxidizing and reacts easily with oxygen and humidity in the air.
rubidium is used in a variety of industries, including the electronics industry and in atomic clock control systems. It is also used as a catalyst in the polymer industry and in the automotive industry. It is used as a spectrophotometer in chemical analysis and can be used to produce rubidium-87, which is a radioactive isotope used in medicine and research.
rubidium is also an important nutrient. It is present in food and is essential for the proper functioning of the heart and nervous system. rubidium is an essential element for the good health of the organism.
rubidium is the most reactive chemical element of the alkalis, and it has an external electronic configuration similar to that of potassium and sodium. It is also the most abundant of the alkalines.
rubidium metal is very malleable and ductile and is more volatile than potassium and sodium. It is soluble in water and turns into Rb+ ions. This ion is strongly oxidizing and reacts easily with oxygen and humidity in the air.
rubidium is used in a variety of industries, including the electronics industry and in atomic clock control systems. It is also used as a catalyst in the polymer industry and in the automotive industry. It is used as a spectrophotometer in chemical analysis and can be used to produce rubidium-87, which is a radioactive isotope used in medicine and research.
rubidium is also an important nutrient. It is present in food and is essential for the proper functioning of the heart and nervous system. rubidium is an essential element for the good health of the organism.
Synthetic
Radioactive
Liquid
Gaseous
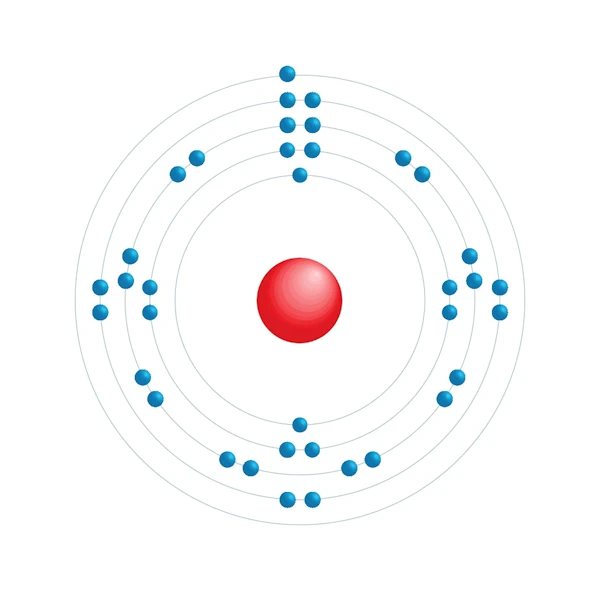
Electronic configuration diagram
| Name | Rubidium |
| Number | 37 |
| Atomic | 85.4678 |
| Symbol | Rb |
| Fusion | 39 |
| Boiling | 688 |
| Density | 1.532 |
| Period | 5 |
| Group | 1 |
| Discovery | 1861 Bunsen and Kirchoff |
| Abundance | 90 |
| Radius | 3 |
| Electronegativity | 0.82 |
| Ionization | 4.1771 |
| Number of isotopes | 20 |
| Electronic configuration | [Kr] 5s1 |
| Oxidation states | 1 |
| Electron by energy level | 2,8,18,8,1 |
| Mineral | Hardness | Density |
| Eveslogite | 5.00 / 5.00 | 2.85 |
| Faizievite | 4.00 / 4.50 | 2.82 |
| Londonite | 8.00 / 8.00 | 3.34 |
| Pautovite | 2.50 / 2.50 | 3.85 |
| Pezzottaite | 8.00 / 8.00 | 2.97 |
| Pollucite | 6.50 / 6.50 | 2.90 |
| Poppiite | 3.36 | |
| Ramanite-(Rb) | ||
| Rubicline | ||
| Telyushenkoite | 6.00 / 6.00 | 2.73 |
| Voloshinite |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





