List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Cl Chlorine
Cl - Chlorine - NON-METAL HALOGEN
Chlorine is a chemical element of the halogen family, with the symbol Cl. It is one of the most reactive elements in the periodic table of the elements and is very common in nature in the chemical form combined under the name of chloride ions. or carbonates.
chlorine is a colorless and very toxic gas. It has a melting point of -100.98°C and a boiling point of 34.6°C. It is one of the most reactive elements in the periodic table of elements.
chlorine is a strong oxidant and can react with a large number of organic and inorganic compounds to form chlorine compounds. It is extremely reactive with metals and alkaline salts, forming chlorides. chlorine has a strong, acrid, characteristic odor.
chlorine is widely used as a strong disinfectant and is used to kill bacteria and viruses in drinking water, waste water and swimming pools. It is also used in the food industry for bleaching products such as sugar, flours and cereals. In addition, chlorine is used as a chemical reagent for the manufacture of polychlorinated biphenyls (PCBs) and chlorofluorocarbons (CFCs).
chlorine is a colorless and very toxic gas. It has a melting point of -100.98°C and a boiling point of 34.6°C. It is one of the most reactive elements in the periodic table of elements.
chlorine is a strong oxidant and can react with a large number of organic and inorganic compounds to form chlorine compounds. It is extremely reactive with metals and alkaline salts, forming chlorides. chlorine has a strong, acrid, characteristic odor.
chlorine is widely used as a strong disinfectant and is used to kill bacteria and viruses in drinking water, waste water and swimming pools. It is also used in the food industry for bleaching products such as sugar, flours and cereals. In addition, chlorine is used as a chemical reagent for the manufacture of polychlorinated biphenyls (PCBs) and chlorofluorocarbons (CFCs).
Synthetic
Radioactive
Liquid
Gaseous
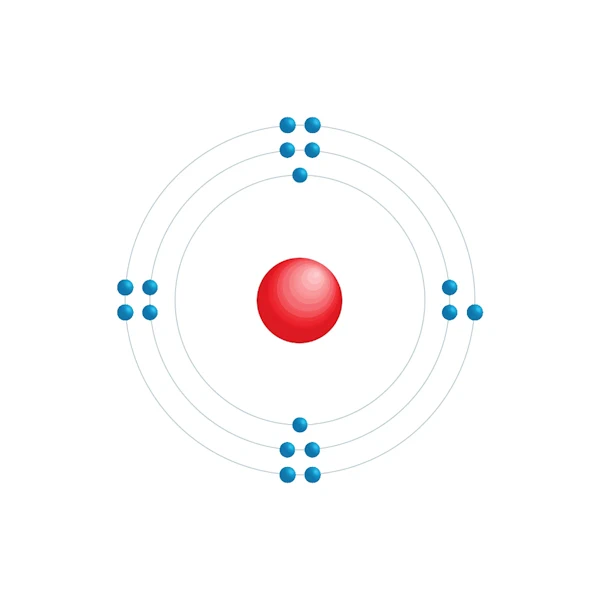
Electronic configuration diagram
| Name | Chlorine |
| Number | 17 |
| Atomic | 35.452 |
| Symbol | Cl |
| Fusion | -101 |
| Boiling | -34.6 |
| Density | 0.003214 |
| Period | 3 |
| Group | 17 |
| Discovery | 1774 Scheele |
| Abundance | 145 |
| Radius | 0.97 |
| Electronegativity | 3.16 |
| Ionization | 12.9676 |
| Number of isotopes | 11 |
| Electronic configuration | [Ne] 3s2 3p5 |
| Oxidation states | -1,1,2,3,4,5,6,7 |
| Electron by energy level | 2,8,7 |
| Mineral | Hardness | Density |
| Abhurite | 2.00 / 2.00 | 4.29 |
| Acmonidesite | ||
| Adranosite | ||
| Adranosite-(Fe) | 2.20 | |
| Adrianite | ||
| Afghanite | 5.50 / 6.00 | 2.55 |
| Aiolosite | 3.59 | |
| Akaganeite | 3.00 | |
| Alexkhomyakovite | ||
| Alforsite | 5.00 / 5.00 | 4.73 |
| Allochalcoselite | 3.00 / 4.00 | 4.65 |
| Alloriite | 5.00 / 5.00 | 2.35 |
| Alluaivite | 5.00 / 6.00 | 2.76 |
| Altisite | 6.00 / 6.00 | 2.64 |
| Ammineite | ||
| Amstallite | 4.00 / 4.00 | 2.40 |
| Anatacamite | 4.00 / 4.50 | |
| Anhydrokainite | ||
| Antarcticite | 2.00 / 3.00 | 1.71 |
| Anthonyite | 2.00 / 2.00 | |
| Aqualite | 4.00 / 5.00 | 2.66 |
| Ardaite | 2.50 / 3.00 | 6.00 |
| Argesite | 2.84 | |
| Arsmirandite | ||
| Arzakite | 2.00 / 2.50 | 7.64 |
| Arzrunite | ||
| Ashburtonite | 4.69 | |
| Asisite | 3.50 / 3.50 | 8.00 |
| Atacamite | 3.00 / 3.50 | 3.76 |
| Atlasovite | 2.00 / 2.50 | 4.20 |
| Aubertite | 2.00 / 3.00 | 1.82 |
| Aurivilliusite | 8.96 | |
| Avdoninite | 3.00 / 3.00 | 3.03 |
| Averievite | 4.00 / 4.00 | 3.54 |
| Backite | 2.00 / 3.00 | 5.57 |
| Balliranoite | ||
| Bandylite | 2.50 / 2.50 | 2.81 |
| Baotite | 6.00 / 6.00 | 4.42 |
| Barahonaite-(Al) | ||
| Barahonaite-(Fe) | 3.03 | |
| Barstowite | 3.00 / 3.00 | 5.50 |
| Belloite | 1.00 / 2.00 | 3.79 |
| Bideauxite | 3.00 / 3.00 | 6.27 |
| Bischofite | 1.50 / 2.00 | 1.56 |
| Bismoclite | 2.00 / 2.50 | 7.72 |
| Bleasdaleite | 2.00 / 2.00 | |
| Blixite | 3.00 / 3.00 | 7.35 |
| Bluebellite | 1.00 / 1.00 | 4.75 |
| Bluelizardite | 2.00 / 2.00 | 3.12 |
| Bobkingite | 3.00 / 3.00 | 3.25 |
| Bobmeyerite | 4.38 | |
| Boleite | 3.00 / 3.50 | 4.80 |
| Boracite | 7.00 / 7.00 | 2.90 |
| Botallackite | 3.60 | |
| Brearleyite | ||
| Brianroulstonite | 5.00 / 5.00 | 1.97 |
| Brokenhillite | 4.00 / 5.00 | 3.00 |
| Brontesite | 2.73 | |
| Burnsite | 1.00 / 1.50 | |
| Buttgenbachite | 3.00 / 3.00 | 3.42 |
| Cadwaladerite | 1.66 | |
| Calclacite | 1.00 | |
| Calomel | 1.50 / 2.00 | 6.40 |
| Calumetite | 2.00 / 2.00 | |
| Capgaronnite | 6.19 | |
| Caracolite | 4.50 / 4.50 | 5.10 |
| Carbokentbrooksite | 5.00 / 5.00 | 3.14 |
| Carnallite | 2.50 / 2.50 | 1.60 |
| Centennialite | ||
| Cerchiaraite-(Al) | 4.50 / 4.50 | 3.64 |
| Cerchiaraite-(Fe) | 4.50 / 4.50 | 3.71 |
| Cerchiaraite-(Mn) | 4.00 / 5.00 | |
| Challacolloite | 2.00 / 3.00 | 4.77 |
| Chambersite | 7.00 / 7.00 | 3.49 |
| Chanabayaite | 2.00 / 2.00 | 1.46 |
| Chelkarite | 1.00 | |
| Chloraluminite | 1.67 | |
| Chlorapatite | 5.00 / 5.00 | |
| Chlorargyrite | 1.00 / 1.50 | 5.55 |
| Chlorartinite | 1.87 | |
| Chlorbartonite | 4.00 / 4.00 | 3.70 |
| Chlorkyuygenite | 5.00 / 5.50 | 2.94 |
| Chlormagaluminite | 1.50 / 2.50 | 1.98 |
| Chlormanganokalite | 2.50 / 2.50 | 2.31 |
| Chlormayenite | ||
| Chloro-potassic-ferro-edenite | ||
| Chlorocalcite | 2.50 / 3.00 | |
| Chloromagnesite | 2.33 | |
| Chloromenite | 1.50 / 2.50 | |
| Chlorothionite | 2.50 / 2.50 | 2.69 |
| Chloroxiphite | 2.50 / 2.50 | 6.76 |
| Choloalite | 3.00 / 3.00 | 6.40 |
| Chrysothallite | 2.97 | |
| Chubarovite | 2.00 / 2.00 | 2.72 |
| Claringbullite | 3.90 | |
| Clinoatacamite | 3.00 / 3.00 | 3.71 |
| Cobaltogordaite | ||
| Comancheite | 2.00 / 2.00 | 7.70 |
| Congolite | 6.50 / 7.50 | 3.58 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





