List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Se Selenium
Se - Selenium - OTHER NO METAL
Selenium is a chemical element of the family of non-metals. It has the chemical symbol Se and atomic number 34. It is a dense off-white solid at room temperature that occurs naturally in the earth's crust. It is slightly heavier than oxygen and combines with other elements such as hydrogen, oxygen and sulfur to form various compounds.
selenium is present in some trace elements and is crucial for human and animal health. It plays an important role in protein metabolism, is necessary for the synthesis of thyroid hormones and, in human and veterinary medicine, is often used for the treatment of diseases caused by viruses, bacteria and fungi. selenium is also used in the food industry for food preservation, in the cosmetics industry for its antioxidant properties, and in the pharmaceutical industry for the manufacture of drugs and nutritional supplements.
selenium is known to be a pyrophore, which means that it ignites spontaneously on contact with oxygen. Due to its toxicity at high concentrations, precautionary measures should be taken when handling it. Additionally, selenium is highly reactive and easily combines with other elements to form various compounds that may have different properties.
selenium is present in some trace elements and is crucial for human and animal health. It plays an important role in protein metabolism, is necessary for the synthesis of thyroid hormones and, in human and veterinary medicine, is often used for the treatment of diseases caused by viruses, bacteria and fungi. selenium is also used in the food industry for food preservation, in the cosmetics industry for its antioxidant properties, and in the pharmaceutical industry for the manufacture of drugs and nutritional supplements.
selenium is known to be a pyrophore, which means that it ignites spontaneously on contact with oxygen. Due to its toxicity at high concentrations, precautionary measures should be taken when handling it. Additionally, selenium is highly reactive and easily combines with other elements to form various compounds that may have different properties.
Synthetic
Radioactive
Liquid
Gaseous
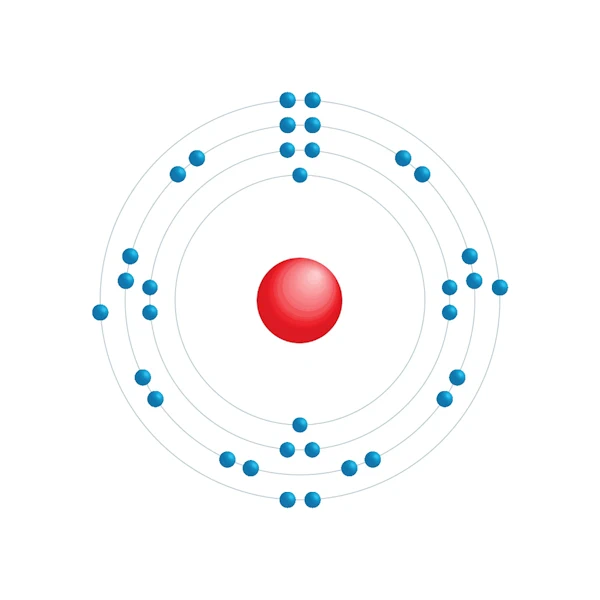
Electronic configuration diagram
| Name | Selenium |
| Number | 34 |
| Atomic | 78.971 |
| Symbol | Se |
| Fusion | 217 |
| Boiling | 685 |
| Density | 4.809 |
| Period | 4 |
| Group | 16 |
| Discovery | 1817 Berzelius |
| Abundance | 0.05 |
| Radius | 1.2 |
| Electronegativity | 2.55 |
| Ionization | 9.7524 |
| Number of isotopes | 20 |
| Electronic configuration | [Ar] 3d10 4s2 4p4 |
| Oxidation states | -2,2,4,6 |
| Electron by energy level | 2,8,18,6 |
| Mineral | Hardness | Density |
| Abramovite | ||
| Achávalite | 2.50 / 2.50 | 6.53 |
| Aguilarite | 2.00 / 3.00 | 7.40 |
| Ahlfeldite | 2.00 / 2.50 | 3.37 |
| Alfredopetrovite | 2.50 / 2.50 | 2.50 |
| Allochalcoselite | 3.00 / 4.00 | 4.65 |
| Antimonselite | 3.50 / 3.50 | 5.88 |
| Athabascaite | 2.50 / 3.00 | 6.00 |
| Babkinite | 2.00 / 2.00 | 8.09 |
| Bambollaite | 3.00 / 3.00 | 5.64 |
| Bellidoite | 1.50 / 2.00 | 7.03 |
| Berzelianite | 2.00 / 2.00 | 6.70 |
| Bohdanowiczite | 3.00 / 3.00 | 7.87 |
| Bornhardtite | 4.00 / 4.00 | |
| Brodtkorbite | 2.50 / 3.00 | 7.77 |
| Bukovite | 2.00 / 2.00 | 7.36 |
| Burnsite | 1.00 / 1.50 | |
| Cadmoselite | 4.00 / 4.00 | 5.66 |
| Carlosruizite | 2.50 / 3.00 | 3.42 |
| Chalcomenite | 2.00 / 2.50 | 3.31 |
| Chaméanite | 4.50 / 4.50 | 6.17 |
| Chloromenite | 1.50 / 2.50 | |
| Chrisstanleyite | 5.00 / 5.00 | 8.31 |
| Clausthalite | 2.50 / 2.50 | 7.60 |
| Clinochalcomenite | 2.00 / 2.00 | 3.28 |
| Cobaltomenite | 2.00 / 2.50 | 3.39 |
| Crerarite | 3.00 / 3.00 | 7.00 |
| Crookesite | 2.50 / 3.00 | 6.90 |
| Cupromakovickyite | 4.00 / 4.50 | 6.78 |
| Demesmaekerite | 3.00 / 4.00 | 5.28 |
| Derriksite | 4.72 | |
| Downeyite | 4.15 | |
| Drysdallite | 1.00 / 1.50 | 6.25 |
| Dzharkenite | 5.00 / 5.00 | 7.34 |
| Eldragónite | 3.50 / 3.50 | |
| Eskebornite | 3.00 / 3.50 | 5.35 |
| Eucairite | 2.50 / 2.50 | 7.60 |
| Favreauite | 3.00 / 3.00 | 4.85 |
| Ferroselite | 6.00 / 6.50 | 7.21 |
| Fischesserite | 2.00 / 2.50 | 9.05 |
| Francisite | 3.00 / 4.00 | 5.00 |
| Freboldite | 2.50 / 3.00 | 7.00 |
| Geffroyite | 2.50 / 2.50 | 5.39 |
| Georgbokiite | 4.00 / 4.00 | |
| Giraudite | 3.50 / 4.00 | 5.75 |
| Grundmannite | 2.00 / 2.50 | 6.58 |
| Guanajuatite | 2.50 / 3.50 | 6.25 |
| Guilleminite | 2.00 / 2.00 | 4.88 |
| Hakite | 5.00 / 5.00 | 6.30 |
| Hansblockite | 2.00 / 2.50 | |
| Hastite | 6.00 / 6.00 | 7.22 |
| Haynesite | 1.50 / 2.00 | 4.10 |
| Ikunolite | 2.00 / 2.00 | 7.80 |
| Ilinskite | 1.50 / 2.00 | 4.08 |
| Jacutingaite | 3.50 / 3.50 | |
| Jagüéite | 5.00 / 5.00 | 8.01 |
| Jolliffeite | 6.00 / 6.50 | 7.10 |
| Jonassonite | 2.50 / 3.00 | |
| Junoite | 3.50 / 4.00 | 6.77 |
| Kalungaite | 7.59 | |
| Kawazulite | 1.50 / 1.50 | 7.79 |
| Kitkaite | 3.50 / 3.50 | 7.19 |
| Klockmannite | 2.00 / 2.50 | 5.99 |
| Krut'aite | 4.00 / 4.00 | 6.00 |
| Kudriavite | 6.58 | |
| Kullerudite | 5.50 / 6.50 | 6.72 |
| Kurilite | ||
| Laitakarite | 2.00 / 2.00 | 8.12 |
| Laphamite | 1.00 / 2.00 | 4.50 |
| Larisaite | 1.00 / 1.00 | |
| Litochlebite | 3.00 / 3.00 | 7.90 |
| Luberoite | 5.00 / 5.50 | 13.02 |
| Mäkinenite | 2.50 / 3.00 | 7.00 |
| Mandarinoite | 2.50 / 2.50 | 2.93 |
| Marthozite | 6.00 / 6.00 | 4.44 |
| Mgriite | 4.50 / 5.00 | 4.00 |
| Miessiite | 2.00 / 2.50 | 10.94 |
| Milotaite | 4.50 / 4.50 | 7.95 |
| Molybdomenite | 3.50 / 3.50 | 7.07 |
| Mozgovaite | 2.50 / 3.50 | |
| Munakataite | 1.50 / 1.50 | 5.53 |
| Mutnovskite | 2.00 / 2.00 | 6.18 |
| Naumannite | 2.50 / 2.50 | 6.50 |
| Nestolaite | 2.50 / 2.50 | 3.16 |
| Nevskite | 3.00 / 3.00 | 7.00 |
| Nicksobolevite | 2.00 / 2.50 | 4.18 |
| Nordströmite | 2.00 / 2.50 | 7.00 |
| Olsacherite | 3.00 / 3.50 | 6.55 |
| Oosterboschite | 5.00 / 5.00 | 8.48 |
| Orlandiite | ||
| Padmaite | 3.00 / 4.00 | 9.00 |
| Palladseite | 4.50 / 5.00 | 8.30 |
| Parageorgbokiite | 3.00 / 4.00 | 4.70 |
| Paraguanajuatite | 2.50 / 3.00 | 6.60 |
| Pauladamsite | 2.00 / 2.00 | 3.54 |
| Pekoite | 2.00 / 3.00 | 6.00 |
| Penroseite | 2.50 / 3.00 | 6.58 |
| Penzhinite | 8.00 | |
| Permingeatite | 4.00 / 4.50 | 5.00 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





