List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Br Bromine
Br - Bromine - NON-METAL HALOGEN
Bromine is a chemical element with symbol Br and atomic number 35, which is found in the halogen column of the Periodic Table of the Elements. It is the fifth and last member of this family, and is located immediately above iodine.
bromine is a metalloid element that has similarities to chlorine and iodine. It is a naturally occurring solid and is very unstable in air and slightly soluble in water. It is pale purple in gaseous state or solid in liquid state. It is very toxic to living organisms and can be easily heated or "roasted" to form monochloride bromine.
bromine is a very reactive element. It readily reacts with chlorine and oxygen to form hydrochloric and hydrobromic acids. It is also very capable of forming organic compounds and has a relatively high boiling point (178°C).
bromine is primarily used in cleaning products, such as disinfectants, due to its antiseptic properties. It is also used in building materials, such as concrete or plastic, to give them a dark purple color and to give them weather resistance. It is also used in the paper and rubber products industry to speed up their drying. Finally, it is also used in odor and air pollutant control systems.
bromine is a metalloid element that has similarities to chlorine and iodine. It is a naturally occurring solid and is very unstable in air and slightly soluble in water. It is pale purple in gaseous state or solid in liquid state. It is very toxic to living organisms and can be easily heated or "roasted" to form monochloride bromine.
bromine is a very reactive element. It readily reacts with chlorine and oxygen to form hydrochloric and hydrobromic acids. It is also very capable of forming organic compounds and has a relatively high boiling point (178°C).
bromine is primarily used in cleaning products, such as disinfectants, due to its antiseptic properties. It is also used in building materials, such as concrete or plastic, to give them a dark purple color and to give them weather resistance. It is also used in the paper and rubber products industry to speed up their drying. Finally, it is also used in odor and air pollutant control systems.
Synthetic
Radioactive
Liquid
Gaseous
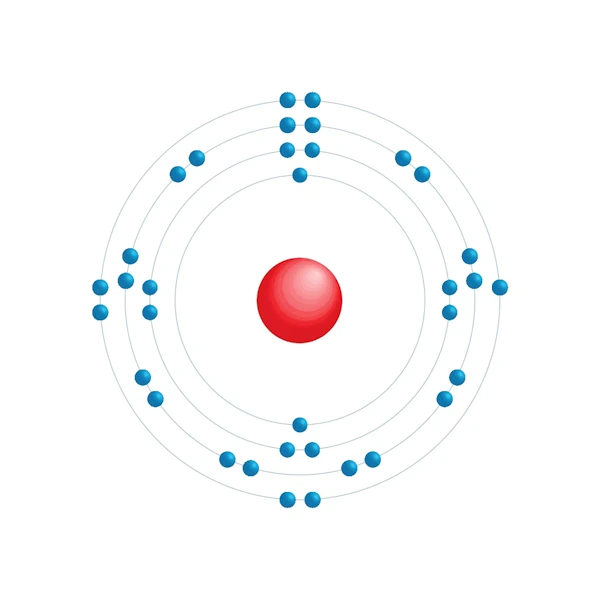
Electronic configuration diagram
| Name | Bromine |
| Number | 35 |
| Atomic | 79.9049 |
| Symbol | Br |
| Fusion | -7.3 |
| Boiling | 58.8 |
| Density | 3.122 |
| Period | 4 |
| Group | 17 |
| Discovery | 1826 Balard |
| Abundance | 2.4 |
| Radius | 1.1 |
| Electronegativity | 2.96 |
| Ionization | 11.8138 |
| Number of isotopes | 19 |
| Electronic configuration | [Ar] 3d10 4s2 4p5 |
| Oxidation states | -1,1,3,4,5,7 |
| Electron by energy level | 2,8,18,7 |
| Mineral | Hardness | Density |
| Arzakite | 2.00 / 2.50 | 7.64 |
| Aurivilliusite | 8.96 | |
| Barlowite | 2.00 / 2.50 | 4.21 |
| Bromargyrite | 1.50 / 2.00 | 5.80 |
| Capgaronnite | 6.19 | |
| Comancheite | 2.00 / 2.00 | 7.70 |
| Demicheleite-(Br) | ||
| Grechishchevite | 2.50 / 2.50 | 7.16 |
| Hephaistosite | ||
| Iltisite | 6.59 | |
| Kadyrelite | 2.50 / 3.00 | 8.00 |
| Kelyanite | 8.51 | |
| Kuzminite | 1.50 / 1.50 | 7.00 |
| Lafossaite | 3.00 / 4.00 | 7.21 |
| Lavrentievite | 2.00 / 2.50 | |
| Lucabindiite | 3.68 | |
| Murdochite | 4.00 / 4.00 | 5.90 |
| Mutnovskite | 2.00 / 2.00 | 6.18 |
| Panichiite | 2.43 | |
| Perroudite | 2.00 / 2.00 | |
| Steropesite | 5.74 | |
| Tedhadleyite | 2.50 / 2.50 | |
| Terlinguacreekite | 2.00 / 3.00 | 9.90 |
| Vasilyevite | 3.00 / 3.00 | 9.57 |
| Vurroite | 6.15 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





