List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Pm Promethium
Pm - Promethium - METAL LANTHANIDES
Promethium (chemical symbol: Pm) is a radioactive chemical element that is found in the lantanide group of metals. It has the atomic number 61 and an atomic weight of 145. It is very rare in nature and must be synthesized to obtain.
Characteristics – promethium has an oxidation state of +3 and occurs as a soft, ductile metal. Its density is 7.26 g/cm3 and its melting temperature is 1100°C.
Properties – promethium is the only chemical element that is totally radioactive. It degrades by producing alpha, beta and gamma rays. It degrades very rapidly and its degradation products are not stable.
Uses – promethium is primarily used as an energy source in special atomic batteries, which power certain medical and space applications. In addition, its radioactivity is used in different types of analytical tests, as this allows specific elements to be identified. Finally, it is used in certain medical studies and radiotherapy applications.
Characteristics – promethium has an oxidation state of +3 and occurs as a soft, ductile metal. Its density is 7.26 g/cm3 and its melting temperature is 1100°C.
Properties – promethium is the only chemical element that is totally radioactive. It degrades by producing alpha, beta and gamma rays. It degrades very rapidly and its degradation products are not stable.
Uses – promethium is primarily used as an energy source in special atomic batteries, which power certain medical and space applications. In addition, its radioactivity is used in different types of analytical tests, as this allows specific elements to be identified. Finally, it is used in certain medical studies and radiotherapy applications.
Synthetic
Radioactive
Liquid
Gaseous
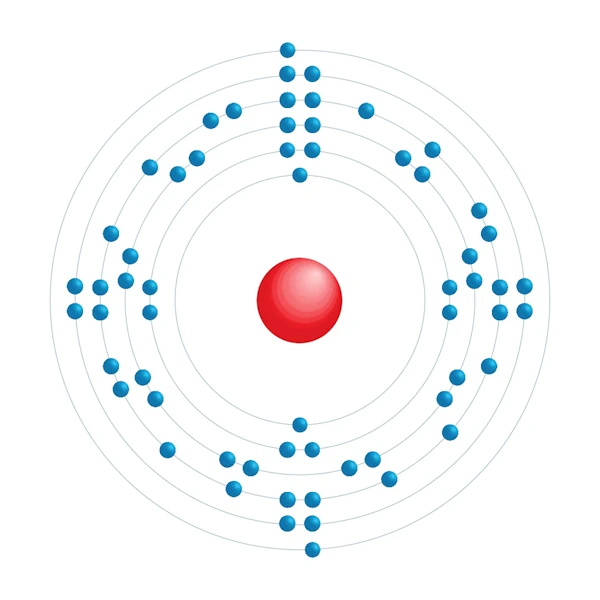
Electronic configuration diagram
| Name | Promethium |
| Number | 61 |
| Atomic | 145 |
| Symbol | Pm |
| Fusion | 1080 |
| Boiling | 2730 |
| Density | 7.26 |
| Period | 6 |
| Group | 0 |
| Discovery | 1945 Marinsky et al. |
| Abundance | 0.001 |
| Radius | 2.6 |
| Electronegativity | 1.13 |
| Ionization | 5.582 |
| Number of isotopes | 14 |
| Electronic configuration | [Xe] 4f5 6s2 |
| Oxidation states | 3 |
| Electron by energy level | 2,8,18,23,8,2 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





