List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Kr Krypton
Kr - Krypton - NO NOBLE GAS METAL
Krypton (Kr) is an inert metallic white chemical element of group 18, or the so-called noble gas group. This element is the lightest of the group, with a krypton atom measuring 84, slightly larger than an argon atom (at 73).
krypton is a very rare gas, as it is only present at 0.00011% in the Earth's atmosphere. It is slightly denser than air, has a very low vapor pressure and is practically colorless. It can be produced in the laboratory from liquid air and is soluble in water.
krypton is inert and is considered a "noble" gas, as it is low reactive and does not react with other elements. Its melting temperature is -157°C and its boiling temperature is -152°C. Its triple point is -152.2°C, -107.7°C and 5.1°C.
krypton is used in fluorescent lamps to improve brightness and durability. It is also used in lasers, medical imaging devices and computers. It is also used to infuse some razor blades, to improve cutting efficiency. krypton is also used in the manufacture of drugs and is an important source of isotopes for the diagnosis and treatment of cancer.
krypton is a very rare gas, as it is only present at 0.00011% in the Earth's atmosphere. It is slightly denser than air, has a very low vapor pressure and is practically colorless. It can be produced in the laboratory from liquid air and is soluble in water.
krypton is inert and is considered a "noble" gas, as it is low reactive and does not react with other elements. Its melting temperature is -157°C and its boiling temperature is -152°C. Its triple point is -152.2°C, -107.7°C and 5.1°C.
krypton is used in fluorescent lamps to improve brightness and durability. It is also used in lasers, medical imaging devices and computers. It is also used to infuse some razor blades, to improve cutting efficiency. krypton is also used in the manufacture of drugs and is an important source of isotopes for the diagnosis and treatment of cancer.
Synthetic
Radioactive
Liquid
Gaseous
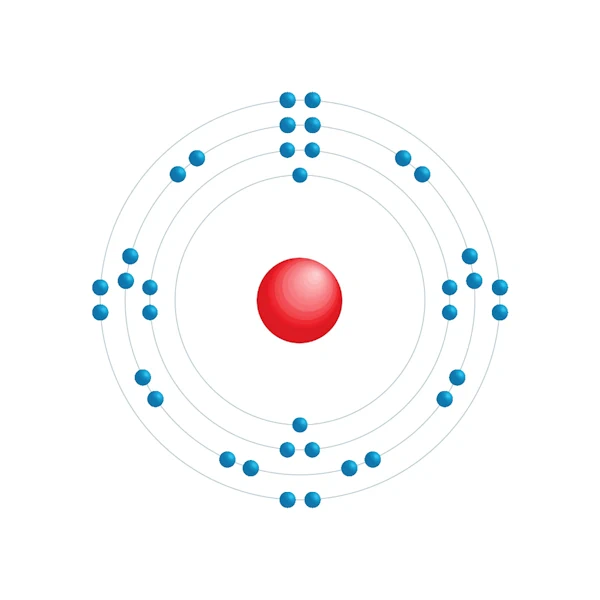
Electronic configuration diagram
| Name | Krypton |
| Number | 36 |
| Atomic | 83.798 |
| Symbol | Kr |
| Fusion | -156.6 |
| Boiling | -152.3 |
| Density | 0.003733 |
| Period | 4 |
| Group | 18 |
| Discovery | 1898 Ramsay and Travers |
| Abundance | 0.001 |
| Radius | 1 |
| Electronegativity | 0 |
| Ionization | 13.9996 |
| Number of isotopes | 23 |
| Electronic configuration | [Ar] 3d10 4s2 4p6 |
| Oxidation states | 2 |
| Electron by energy level | 2,8,18,8 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





