List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
Pb - Lead - POOR METAL
Synthetic
Radioactive
Liquid
Gaseous
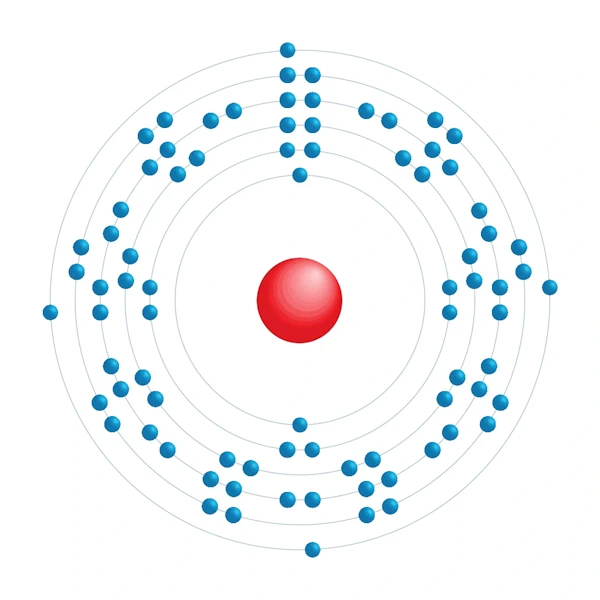
Electronic configuration diagram
| Name | Lead |
| Number | 82 |
| Atomic | 207.2 |
| Symbol | Pb |
| Fusion | 327.5 |
| Boiling | 1740 |
| Density | 11.342 |
| Period | 6 |
| Group | 14 |
| Discovery | 0 Prehistoric |
| Abundance | 14 |
| Radius | 1.8 |
| Electronegativity | 2.33 |
| Ionization | 7.4167 |
| Number of isotopes | 29 |
| Electronic configuration | [Xe] 4f14 5d10 6s2 6p2 |
| Oxidation states | -4,2,4 |
| Electron by energy level | 2,8,18,32,18,4 |
| Mineral | Hardness | Density |
| Abramovite | ||
| Acmonidesite | ||
| Agaite | 6.99 | |
| Agardite-(Nd) | 3.00 / 4.00 | 3.72 |
| Aikinite | 2.00 / 2.50 | 6.10 |
| Alamosite | 4.50 / 4.50 | 6.49 |
| Aleksite | 2.50 / 2.50 | 7.00 |
| Allochalcoselite | 3.00 / 4.00 | 4.65 |
| Almeidaite | ||
| Altaite | 2.50 / 2.50 | 8.10 |
| Andorite IV | 3.50 / 3.50 | 5.00 |
| Andorite VI | 3.50 / 3.50 | 5.00 |
| Andreadiniite | ||
| Andychristyite | 2.00 / 3.00 | 6.30 |
| Ángelaite | ||
| Anglesite | 2.50 / 3.00 | 6.30 |
| Anyuiite | 3.50 / 3.50 | 13.00 |
| Arapovite | 5.50 / 6.00 | 3.43 |
| Aravaipaite | 2.00 / 2.00 | 6.00 |
| Arcubisite | ||
| Ardaite | 2.50 / 3.00 | 6.00 |
| Argentobaumhauerite | 3.00 / 3.00 | 5.31 |
| Argentoliveingite | ||
| Arrojadite-(BaFe) | 3.54 | |
| Arrojadite-(PbFe) | 4.00 / 5.00 | |
| Arrojadite-(SrFe) | ||
| Arsenbrackebuschite | 4.50 / 4.50 | 6.54 |
| Arsendescloizite | 4.00 / 4.00 | 6.57 |
| Arseniopleite | 3.50 / 3.50 | 4.22 |
| Arsenquatrandorite | ||
| Arsentsumebite | 4.00 / 5.00 | 6.46 |
| Artroeite | 2.50 / 2.50 | 5.36 |
| Arzrunite | ||
| Aschamalmite | 3.50 / 3.50 | 7.27 |
| Ashburtonite | 4.69 | |
| Asisite | 3.50 / 3.50 | 8.00 |
| Asselbornite | 3.00 / 3.00 | |
| Babkinite | 2.00 / 2.00 | 8.09 |
| Backite | 2.00 / 3.00 | 5.57 |
| Bairdite | ||
| Barikaite | 5.34 | |
| Barstowite | 3.00 / 3.00 | 5.50 |
| Bartelkeite | 4.00 / 4.00 | 4.97 |
| Barysilite | 3.00 / 3.00 | 6.11 |
| Baumhauerite | 3.00 / 3.00 | 5.33 |
| Baumhauerite II | 3.00 / 3.00 | 5.00 |
| Bayldonite | 4.50 / 4.50 | 5.50 |
| Beaverite-(Cu) | ||
| Beaverite-(Zn) | ||
| Benauite | 3.50 / 3.50 | 3.65 |
| Benavidesite | 2.50 / 2.50 | 5.60 |
| Benjaminite | 3.50 / 3.50 | 6.70 |
| Bernalite | 4.00 / 4.00 | 3.32 |
| Bernarlottiite | ||
| Berryite | 3.50 / 3.50 | 6.70 |
| Betekhtinite | 3.00 / 3.00 | 5.96 |
| Beudantite | 4.00 / 4.00 | 4.10 |
| Beyerite | 2.00 / 3.00 | 6.56 |
| Bezsmertnovite | 4.50 / 4.50 | 16.30 |
| Bideauxite | 3.00 / 3.00 | 6.27 |
| Bilibinskite | 5.00 / 5.00 | 14.27 |
| Bindheimite | 4.00 / 5.00 | 4.60 |
| Bismutopyrochlore | 5.00 / 5.00 | 4.97 |
| Blixite | 3.00 / 3.00 | 7.35 |
| Bobmeyerite | 4.38 | |
| Bogdanovite | 4.00 / 4.50 | |
| Boleite | 3.00 / 3.50 | 4.80 |
| Borishanskiite | 4.00 / 4.00 | 10.00 |
| Borodaevite | 3.50 / 3.50 | 7.90 |
| Boscardinite | 3.00 | |
| Boulangerite | 2.50 / 2.50 | 5.70 |
| Bournonite | 3.00 / 3.00 | 5.70 |
| Brackebuschite | 4.00 / 5.00 | 6.05 |
| Brendelite | 4.50 / 4.50 | 6.83 |
| Britvinite | 3.00 / 3.00 | 5.51 |
| Brontesite | 2.73 | |
| Brumadoite | 1.00 / 1.00 | 4.77 |
| Buckhornite | 2.50 / 2.50 | 8.00 |
| Burckhardtite | 2.00 / 2.00 | 4.96 |
| Bushmakinite | 3.00 / 3.50 | 6.22 |
| Calcioaravaipaite | 2.50 / 2.50 | 4.85 |
| Calciouranoite | 4.00 / 4.00 | 4.62 |
| Calderónite | 3.00 / 4.00 | 6.05 |
| Caledonite | 2.50 / 3.00 | 5.70 |
| Cannizzarite | 2.50 / 2.50 | 6.70 |
| Caracolite | 4.50 / 4.50 | 5.10 |
| Carducciite | 2.50 / 3.00 | 5.56 |
| Carminite | 3.50 / 3.50 | 5.03 |
| Caryinite | 4.00 / 4.00 | 4.29 |
| Cassedanneite | 3.50 / 3.50 | 6.00 |
| Cechite | 4.50 / 5.00 | 5.88 |
| Cerussite | 3.00 / 3.50 | 6.50 |
| Cesàrolite | 4.50 / 4.50 | 5.29 |
| Cesplumtantite | 7.00 / 7.00 | 6.00 |
| Chabournéite | 3.00 / 3.50 | 5.10 |
| Challacolloite | 2.00 / 3.00 | 4.77 |
| Changbaiite | 5.50 / 5.50 | 6.48 |
| Chekhovichite | 4.00 / 4.00 | 6.88 |
| Chenite | 2.50 / 2.50 | 5.98 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





