List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Al Aluminum
Al - Aluminum - POOR METAL
Aluminum is the chemical element with atomic number 13. It is a soft, greyish-white metal with an estimated hardness of 2.5 to 3 on the Mohs scale. It is the third most abundant element in the earth's crust, after oxygen and silicon.
aluminum is highly reactive. It oxidizes rapidly in a humid environment to form a protective coating that prevents it from being attacked by oxygen and humidity. It is very ductile and malleable and can be worked and formed into other products using industrial techniques such as casting, forging and extrusion. It easily combines with other elements to form alloys, which are used in many different products.
aluminum is one of the most widely used metals due to its lightness and resistance to corrosion. It is used in many industrial applications, including building materials, food products, packaging, conveyors, electronic equipment, household appliances and vehicles. It is also used in many alloys, which are used for their corrosion resistance and ease of processing.
aluminum is highly reactive. It oxidizes rapidly in a humid environment to form a protective coating that prevents it from being attacked by oxygen and humidity. It is very ductile and malleable and can be worked and formed into other products using industrial techniques such as casting, forging and extrusion. It easily combines with other elements to form alloys, which are used in many different products.
aluminum is one of the most widely used metals due to its lightness and resistance to corrosion. It is used in many industrial applications, including building materials, food products, packaging, conveyors, electronic equipment, household appliances and vehicles. It is also used in many alloys, which are used for their corrosion resistance and ease of processing.
Synthetic
Radioactive
Liquid
Gaseous
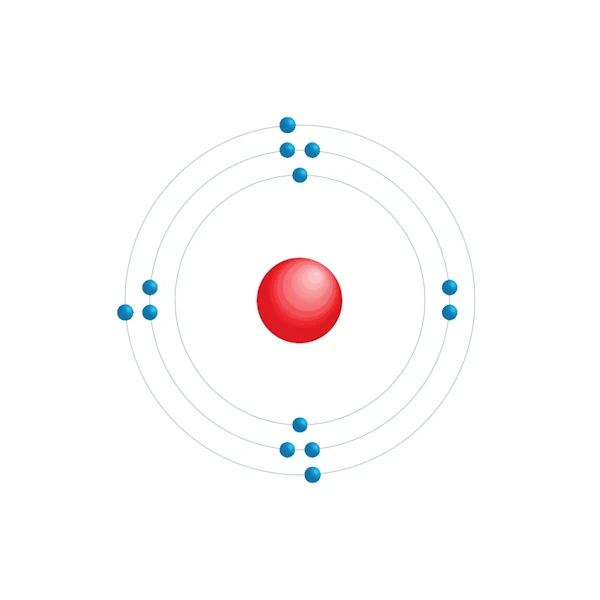
Electronic configuration diagram
| Name | Aluminum |
| Number | 13 |
| Atomic | 26.9815385 |
| Symbol | Al |
| Fusion | 660.5 |
| Boiling | 2467 |
| Density | 2.698 |
| Period | 3 |
| Group | 13 |
| Discovery | 1827 Wshler |
| Abundance | 82300 |
| Radius | 1.8 |
| Electronegativity | 1.61 |
| Ionization | 5.9858 |
| Number of isotopes | 8 |
| Electronic configuration | [Ne] 3s2 3p1 |
| Oxidation states | 1,3 |
| Electron by energy level | 2,8,3 |
| Mineral | Hardness | Density |
| Abswurmbachite | 6.50 / 6.50 | 4.96 |
| Abuite | ||
| Actinolite | 5.50 / 5.50 | 2.98 |
| Acuminite | 3.50 / 3.50 | 3.30 |
| Adachiite | ||
| Addibischoffite | ||
| Adranosite | ||
| Adrianite | ||
| Aenigmatite | 5.00 / 6.00 | 3.74 |
| Aerinite | 3.00 / 3.00 | 2.48 |
| Afghanite | 5.50 / 6.00 | 2.55 |
| Afmite | ||
| Aheylite | 5.00 / 6.00 | 2.85 |
| Ajoite | 2.96 | |
| Akatoreite | 6.00 / 6.00 | 3.48 |
| Akdalaite | 7.00 / 7.00 | 3.68 |
| Alarsite | 3.00 / 3.00 | 3.32 |
| Albite | 7.00 / 7.00 | 2.61 |
| Aldermanite | 2.00 / 2.00 | 2.00 |
| Alfredopetrovite | 2.50 / 2.50 | 2.50 |
| Aliettite | 1.00 / 2.00 | |
| Allanite-(Ce) | 5.50 / 5.50 | 3.30 |
| Allanite-(La) | 6.00 / 6.00 | 3.93 |
| Allanite-(Nd) | ||
| Allanite-(Y) | 5.50 / 5.50 | 3.30 |
| Allanpringite | 3.00 / 3.00 | 2.54 |
| Allendeite | 4.84 | |
| Allophane | 3.00 / 3.00 | 1.90 |
| Alloriite | 5.00 / 5.00 | 2.35 |
| Almandine | 7.00 / 8.00 | 4.09 |
| Almarudite | 6.00 / 6.00 | 2.71 |
| Alnaperbøeite-(Ce) | ||
| Althupite | 3.50 / 4.00 | 3.90 |
| Altisite | 6.00 / 6.00 | 2.64 |
| Alum-(K) | 2.00 / 2.00 | 1.76 |
| Alum-(Na) | 3.00 / 3.00 | 1.67 |
| Aluminite | 1.00 / 1.00 | 1.66 |
| Aluminium | 2.00 / 3.50 | 2.00 |
| Alumino-ferrobarroisite | ||
| Alumino-ferrohornblende | ||
| Alumino-ferrotschermakite | ||
| Alumino-ferrowinchite | ||
| Alumino-magnesiohornblende | ||
| Alumino-magnesiotaramite | ||
| Alumino-ottoliniite | ||
| Aluminobarroisite | 5.00 / 6.00 | 2.94 |
| Aluminoceladonite | ||
| Aluminocerite-(Ce) | 5.00 / 5.00 | 4.68 |
| Aluminocopiapite | 2.50 / 3.00 | 2.00 |
| Aluminocoquimbite | 2.04 | |
| Aluminokatophorite | 5.00 / 6.00 | |
| Aluminomagnesiohulsite | 6.00 / 6.00 | 3.84 |
| Aluminopyracmonite | 2.14 | |
| Aluminotschermakite | 5.00 / 6.00 | |
| Aluminowinchite | ||
| Alumoåkermanite | 4.00 / 5.00 | 3.00 |
| Alumohydrocalcite | 2.50 / 2.50 | 2.23 |
| Alumoklyuchevskite | 2.00 / 2.00 | 3.10 |
| Alumotantite | 7.50 / 7.50 | 7.00 |
| Alumotungstite | ||
| Alumovesuvianite | ||
| Alunite | 3.50 / 4.00 | 2.59 |
| Alunogen | 1.50 / 2.00 | 1.65 |
| Alvanite | 3.00 / 3.50 | 2.41 |
| Amazonite | 6.00 / 6.50 | 2.56 |
| Amblygonite | 5.50 / 6.00 | 2.98 |
| Amesite | 2.50 / 3.00 | 2.77 |
| Amicite | 5.00 / 5.50 | 2.06 |
| Ammonioalunite | 2.00 / 3.00 | 2.40 |
| Ammonioleucite | 5.50 / 6.00 | 2.29 |
| Ammoniomagnesiovoltaite | ||
| Amstallite | 4.00 / 4.00 | 2.40 |
| Analcime | 5.00 / 5.00 | 2.30 |
| Anandite | 3.00 / 4.00 | 3.94 |
| Andalusite | 6.50 / 7.00 | 3.13 |
| Andesine | 7.00 / 7.00 | 2.66 |
| Angastonite | 2.00 / 2.00 | 2.47 |
| Ankinovichite | 2.50 / 3.00 | 2.48 |
| Annite | 2.50 / 3.00 | 3.17 |
| Anorthite | 6.00 / 6.00 | 2.72 |
| Anorthoclase | 6.00 / 6.00 | 2.57 |
| Anthoinite | 1.00 / 1.00 | 4.80 |
| Apjohnite | 1.50 / 2.00 | 1.80 |
| Aquamarine | 7.50 / 8.00 | 2.70 |
| Arakiite | 3.00 / 4.00 | |
| Arangasite | 1.00 / 2.00 | 2.00 |
| Aravaipaite | 2.00 / 2.00 | 6.00 |
| Ardennite-(As) | 6.00 / 7.00 | 3.62 |
| Ardennite-(V) | 6.00 / 7.00 | 3.55 |
| Armbrusterite | 3.50 / 3.50 | 2.78 |
| Armenite | 7.50 / 7.50 | 2.76 |
| Arrojadite-(BaFe) | 3.54 | |
| Arrojadite-(BaNa) | ||
| Arrojadite-(KFe) | 5.00 / 5.00 | 3.50 |
| Arrojadite-(KNa) | ||
| Arrojadite-(NaFe) | ||
| Arrojadite-(PbFe) | 4.00 / 5.00 | |
| Arrojadite-(SrFe) | ||
| Arsenocrandallite | 5.50 / 5.50 | 3.25 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





