List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
Ne - Neon - NO NOBLE GAS METAL
Neon is a chemical element, also called noble gas, which has the symbol Ne and the atomic number 10 on the periodic table of elements. Its electron configuration is 1s22s22p63s1. This means that there are two electrons in the 1s shell and six electrons in the 2p shell.
neon is a colorless and odorless inert gas at room temperature and pressure. It is very weakly reactive and is the most stable of the noble gases. Its melting point is -248.6°C and its boiling point is -246.1°C.
neon is widely used in industry and neon lights are offered in liquid or gaseous form. It is used for making neon lights and can be mixed with other gases, such as argon, to produce different colors. It is also used as a fill gas in vacuum tubes and in industrial illumination and street lighting products.
neon is also used in medicine and scientific research, as its radioactive isotope Ne-21 is used as a radioactive tracer. neon also has applications in lasers, radio astronomy and medical imaging. It can also be used to provide a type of cold in industrial applications.
neon is a colorless and odorless inert gas at room temperature and pressure. It is very weakly reactive and is the most stable of the noble gases. Its melting point is -248.6°C and its boiling point is -246.1°C.
neon is widely used in industry and neon lights are offered in liquid or gaseous form. It is used for making neon lights and can be mixed with other gases, such as argon, to produce different colors. It is also used as a fill gas in vacuum tubes and in industrial illumination and street lighting products.
neon is also used in medicine and scientific research, as its radioactive isotope Ne-21 is used as a radioactive tracer. neon also has applications in lasers, radio astronomy and medical imaging. It can also be used to provide a type of cold in industrial applications.
Synthetic
Radioactive
Liquid
Gaseous
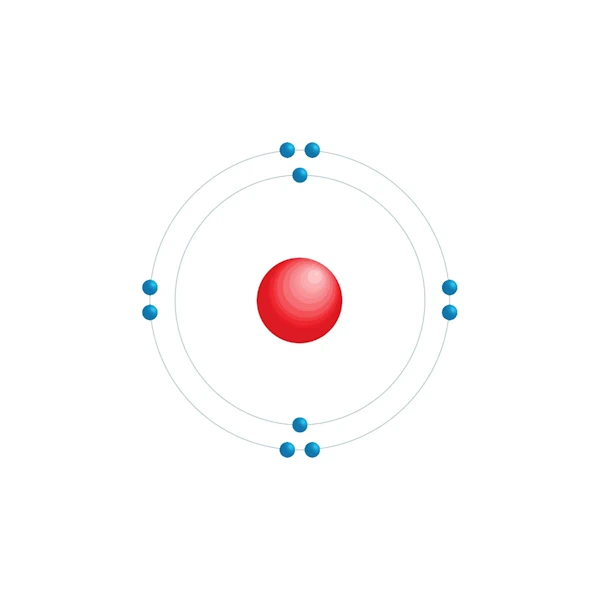
Electronic configuration diagram
| Name | Neon |
| Number | 10 |
| Atomic | 20.1797 |
| Symbol | Ne |
| Fusion | -248.7 |
| Boiling | -246.1 |
| Density | 0.0008999 |
| Period | 2 |
| Group | 18 |
| Discovery | 1898 Ramsay and Travers |
| Abundance | 0.005 |
| Radius | 0.51 |
| Electronegativity | 0 |
| Ionization | 21.5645 |
| Number of isotopes | 8 |
| Electronic configuration | [He] 2s2 2p6 |
| Oxidation states | 0 |
| Electron by energy level | 2,8 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





