List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
Mt meitnerium
Mt - meitnerium - UNCLASSIFIED
Meitnerium is a synthetic chemical element with the symbol Mt and atomic number 109.
Although it does not occur naturally in the Earth's crust, it attracts the interest of researchers and mineralogy enthusiasts due to its unique properties. Discovered in 1982, meitnerium pays tribute to Lise Meitner, a pioneering nuclear physicist.
As a member of the transition metal family, meitnerium is mainly studied in the laboratory for its theoretical characteristics and interactions with other elements.
Although it does not occur naturally in the Earth's crust, it attracts the interest of researchers and mineralogy enthusiasts due to its unique properties. Discovered in 1982, meitnerium pays tribute to Lise Meitner, a pioneering nuclear physicist.
As a member of the transition metal family, meitnerium is mainly studied in the laboratory for its theoretical characteristics and interactions with other elements.
Synthetic
Radioactive
Liquid
Gaseous
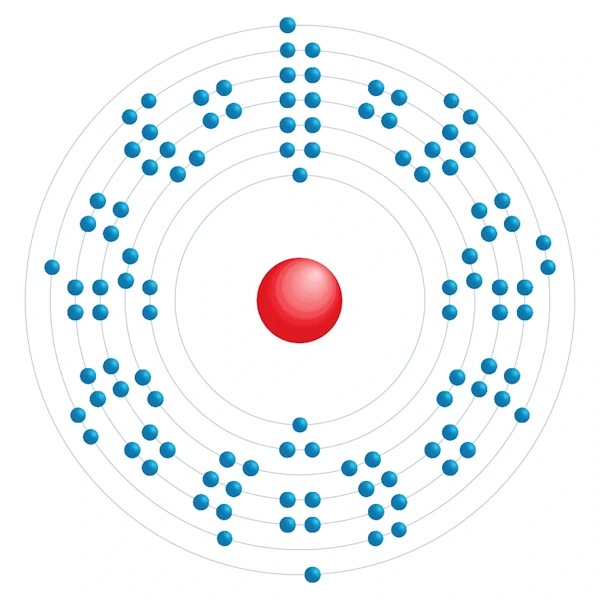
Electronic configuration diagram
| Name | meitnerium |
| Number | 109 |
| Atomic | 278 |
| Symbol | Mt |
| Fusion | 0 |
| Boiling | 0 |
| Density | 37.4 |
| Period | 7 |
| Group | 9 |
| Discovery | 1982 GSI |
| Abundance | 0 |
| Radius | 0 |
| Electronegativity | 0 |
| Ionization | 0 |
| Number of isotopes | 0 |
| Electronic configuration | [Rn] 5f14 6d7 7s2 |
| Oxidation states | 0 |
| Electron by energy level | 2,8,18,32,32,15,2 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





