List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
H Hydrogen
H - Hydrogen - OTHER NO METAL
Hydrogen (symbol H) is the main chemical element of the alkali metal group. It is the lightest and most abundant of all the elements. It is a colorless, odorless and tasteless gas, which easily combines with other elements.
hydrogen is the lightest of the chemical elements, having an atomic weight of 1.00794 g/mol. It is very responsive and can combine with almost any other element. It has an electronic structure 2,
hydrogen is highly flammable and can combine easily with other elements, forming various chemical compounds. Due to its high reactivity, it is very volatile and tends to evaporate. It is very light and is in a gaseous state at room temperature.
hydrogen is the primary fuel used in rockets and space vehicles. It is also used to produce industrial products such as ammonia, petroleum products and chemicals. It is widely used in industry as a fuel for boilers and furnaces, and it is also used in hydrogen fuel engines. Additionally, it is used to generate electricity using fuel cells.
hydrogen is the lightest of the chemical elements, having an atomic weight of 1.00794 g/mol. It is very responsive and can combine with almost any other element. It has an electronic structure 2,
hydrogen is highly flammable and can combine easily with other elements, forming various chemical compounds. Due to its high reactivity, it is very volatile and tends to evaporate. It is very light and is in a gaseous state at room temperature.
hydrogen is the primary fuel used in rockets and space vehicles. It is also used to produce industrial products such as ammonia, petroleum products and chemicals. It is widely used in industry as a fuel for boilers and furnaces, and it is also used in hydrogen fuel engines. Additionally, it is used to generate electricity using fuel cells.
Synthetic
Radioactive
Liquid
Gaseous
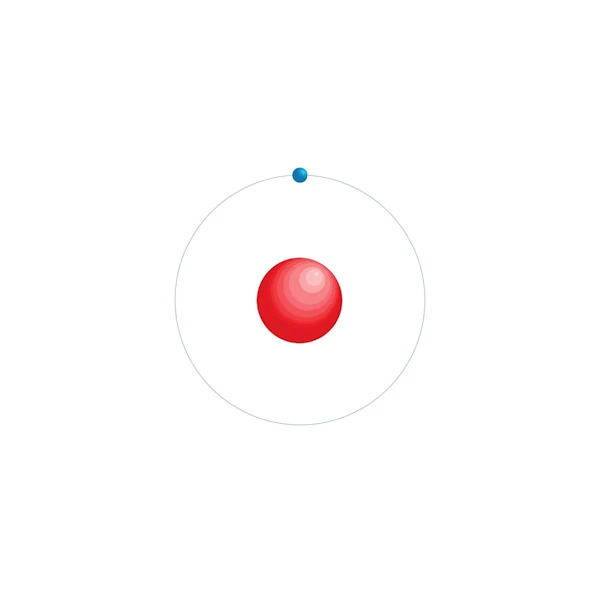
Electronic configuration diagram
| Name | Hydrogen |
| Number | 1 |
| Atomic | 1.0082 |
| Symbol | H |
| Fusion | -259.1 |
| Boiling | -252.9 |
| Density | 8.988E-5 |
| Period | 1 |
| Group | 1 |
| Discovery | 1766 Cavendish |
| Abundance | 1400 |
| Radius | 0.79 |
| Electronegativity | 2.2 |
| Ionization | 13.5984 |
| Number of isotopes | 3 |
| Electronic configuration | 1s1 |
| Oxidation states | -1,1 |
| Electron by energy level | 1 |
| Mineral | Hardness | Density |
| Abelsonite | 2.00 / 2.50 | 1.45 |
| Abernathyite | 2.00 / 3.00 | 3.31 |
| Abhurite | 2.00 / 2.00 | 4.29 |
| Acetamide | 1.00 / 1.50 | 1.17 |
| Acmonidesite | ||
| Actinolite | 5.50 / 5.50 | 2.98 |
| Acuminite | 3.50 / 3.50 | 3.30 |
| Adachiite | ||
| Adamite | 3.50 / 3.50 | 4.30 |
| Adamsite-(Y) | 3.00 / 3.00 | |
| Adelite | 5.00 / 5.00 | 3.73 |
| Admontite | 2.00 / 3.00 | 1.82 |
| Adolfpateraite | 2.00 / 2.00 | 4.24 |
| Adranosite | ||
| Adranosite-(Fe) | 2.20 | |
| Aerinite | 3.00 / 3.00 | 2.48 |
| Aeschynite-(Ce) | 5.00 / 6.00 | 5.19 |
| Aeschynite-(Nd) | 5.00 / 6.00 | 4.60 |
| Aeschynite-(Y) | 5.00 / 6.00 | 4.85 |
| Afghanite | 5.50 / 6.00 | 2.55 |
| Afmite | ||
| Afwillite | 3.00 / 3.00 | 2.62 |
| Agaite | 6.99 | |
| Agardite-(Ce) | 3.00 / 3.00 | 3.72 |
| Agardite-(La) | 3.00 / 4.00 | 3.72 |
| Agardite-(Nd) | 3.00 / 4.00 | 3.72 |
| Agardite-(Y) | 3.00 / 4.00 | 3.66 |
| Agrinierite | 5.62 | |
| Aheylite | 5.00 / 6.00 | 2.85 |
| Ahlfeldite | 2.00 / 2.50 | 3.37 |
| Ajoite | 2.96 | |
| Akaganeite | 3.00 | |
| Akatoreite | 6.00 / 6.00 | 3.48 |
| Akdalaite | 7.00 / 7.00 | 3.68 |
| Aklimaite | ||
| Akrochordite | 3.50 / 3.50 | 3.19 |
| Aksaite | 2.50 / 2.50 | 1.99 |
| Albertiniite | 2.46 | |
| Albrechtschraufite | 2.00 / 3.00 | 2.60 |
| Alcaparrosaite | 4.00 / 4.00 | 2.81 |
| Aldermanite | 2.00 / 2.00 | 2.00 |
| Aldridgeite | 3.00 / 3.00 | 3.33 |
| Alexkhomyakovite | ||
| Alflarsenite | 4.00 / 4.00 | 2.61 |
| Alfredopetrovite | 2.50 / 2.50 | 2.50 |
| Alfredstelznerite | ||
| Aliettite | 1.00 / 2.00 | |
| Allactite | 4.50 / 4.50 | 3.00 |
| Allanite-(Ce) | 5.50 / 5.50 | 3.30 |
| Allanite-(La) | 6.00 / 6.00 | 3.93 |
| Allanite-(Nd) | ||
| Allanite-(Y) | 5.50 / 5.50 | 3.30 |
| Allanpringite | 3.00 / 3.00 | 2.54 |
| Alleghanyite | 6.00 / 5.00 | 4.00 |
| Allophane | 3.00 / 3.00 | 1.90 |
| Alloriite | 5.00 / 5.00 | 2.35 |
| Alluaivite | 5.00 / 6.00 | 2.76 |
| Almeidaite | ||
| Alnaperbøeite-(Ce) | ||
| Alpersite | 2.50 / 2.50 | |
| Alsakharovite-Zn | 5.00 / 5.00 | 2.90 |
| Althausite | 3.50 / 3.50 | 2.97 |
| Althupite | 3.50 / 4.00 | 3.90 |
| Alum-(K) | 2.00 / 2.00 | 1.76 |
| Alum-(Na) | 3.00 / 3.00 | 1.67 |
| Aluminite | 1.00 / 1.00 | 1.66 |
| Alumino-ferrobarroisite | ||
| Alumino-ferrohornblende | ||
| Alumino-ferrotschermakite | ||
| Alumino-ferrowinchite | ||
| Alumino-magnesiohornblende | ||
| Alumino-magnesiotaramite | ||
| Alumino-ottoliniite | ||
| Aluminobarroisite | 5.00 / 6.00 | 2.94 |
| Aluminoceladonite | ||
| Aluminocerite-(Ce) | 5.00 / 5.00 | 4.68 |
| Aluminocopiapite | 2.50 / 3.00 | 2.00 |
| Aluminocoquimbite | 2.04 | |
| Aluminokatophorite | 5.00 / 6.00 | |
| Aluminopyracmonite | 2.14 | |
| Aluminotschermakite | 5.00 / 6.00 | |
| Aluminowinchite | ||
| Alumohydrocalcite | 2.50 / 2.50 | 2.23 |
| Alumotungstite | ||
| Alumovesuvianite | ||
| Alunite | 3.50 / 4.00 | 2.59 |
| Alunogen | 1.50 / 2.00 | 1.65 |
| Alvanite | 3.00 / 3.50 | 2.41 |
| Alwilkinsite-(Y) | 2.00 / 2.50 | 3.37 |
| Amakinite | 3.50 / 4.00 | 2.93 |
| Amarantite | 2.50 / 2.50 | 2.19 |
| Amarillite | 2.50 / 3.00 | 2.19 |
| Amber | 2.00 / 2.50 | 1.10 |
| Amblygonite | 5.50 / 6.00 | 2.98 |
| Ambrinoite | 3.31 | |
| Ameghinite | 2.00 / 3.00 | 2.02 |
| Amesite | 2.50 / 3.00 | 2.77 |
| Amicite | 5.00 / 5.50 | 2.06 |
| Aminoffite | 5.50 / 5.50 | 2.94 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





