List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
mineralogy
elements
As Arsenic
As - Arsenic - METALLOIDS
Arsenic is a non-metallic chemical element with symbol At and atomic number 33 that is part of the metalloid family. It is often in its trivalent or pentavalent form and is present in the earth's crust, subsoil and some waters. In its pure state, arsenic is an off-white, grey-yellow, slightly shiny solid. Its solubility is very low in water and very reactive with acids.
arsenic is very toxic and the fact that trace amounts can be fatal makes it an extremely dangerous natural poison. In the Middle Ages, arsenic was used in the manufacture of patent dyes for home dyeing and is known as "color poison". The toxicity of arsenic has long been recognized and in Europe it was known as the "poison of kings", as many monarchs were thought to have been poisoned with it.
Despite its chloride, arsenic is used as a food additive and implicated in the manufacture of matches, agricultural products such as herbicides and insecticides, and in the treatment of wood. It is also used in the battery industry, electronics and pyrotechnics. Natural arsenic is also used in the semiconductor industry and arsenic compounds are used to make products such as plastics and paints.
arsenic is very toxic and the fact that trace amounts can be fatal makes it an extremely dangerous natural poison. In the Middle Ages, arsenic was used in the manufacture of patent dyes for home dyeing and is known as "color poison". The toxicity of arsenic has long been recognized and in Europe it was known as the "poison of kings", as many monarchs were thought to have been poisoned with it.
Despite its chloride, arsenic is used as a food additive and implicated in the manufacture of matches, agricultural products such as herbicides and insecticides, and in the treatment of wood. It is also used in the battery industry, electronics and pyrotechnics. Natural arsenic is also used in the semiconductor industry and arsenic compounds are used to make products such as plastics and paints.
Synthetic
Radioactive
Liquid
Gaseous
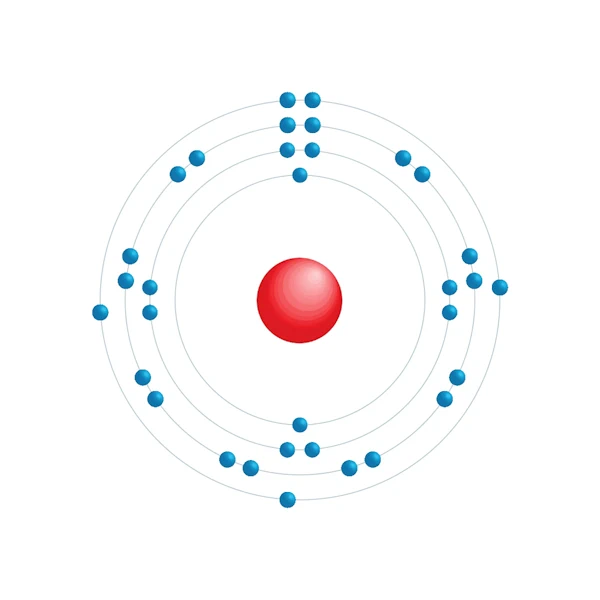
Electronic configuration diagram
| Name | Arsenic |
| Number | 33 |
| Atomic | 74.921595 |
| Symbol | As |
| Fusion | 613 |
| Boiling | 613 |
| Density | 5.776 |
| Period | 4 |
| Group | 15 |
| Discovery | 1250 Albertus Magnus |
| Abundance | 1.8 |
| Radius | 1.3 |
| Electronegativity | 2.18 |
| Ionization | 9.7886 |
| Number of isotopes | 14 |
| Electronic configuration | [Ar] 3d10 4s2 4p3 |
| Oxidation states | -3,2,3,5 |
| Electron by energy level | 2,8,18,5 |
| Mineral | Hardness | Density |
| Abernathyite | 2.00 / 3.00 | 3.31 |
| Adamite | 3.50 / 3.50 | 4.30 |
| Adelite | 5.00 / 5.00 | 3.73 |
| Aerugite | 4.00 / 4.00 | 5.85 |
| Agardite-(Ce) | 3.00 / 3.00 | 3.72 |
| Agardite-(La) | 3.00 / 4.00 | 3.72 |
| Agardite-(Nd) | 3.00 / 4.00 | 3.72 |
| Agardite-(Y) | 3.00 / 4.00 | 3.66 |
| Akrochordite | 3.50 / 3.50 | 3.19 |
| Aktashite | 3.50 / 3.50 | 5.50 |
| Alacránite | 1.50 / 1.50 | 3.40 |
| Alarsite | 3.00 / 3.00 | 3.32 |
| Algodonite | 4.00 / 4.00 | 8.38 |
| Allactite | 4.50 / 4.50 | 3.00 |
| Alloclasite | 5.00 / 5.00 | 6.17 |
| Ambrinoite | 3.31 | |
| Anduoite | 7.00 / 7.00 | 8.00 |
| Andyrobertsite | 3.00 / 3.00 | 4.01 |
| Angelellite | 5.50 / 5.50 | 4.87 |
| Annabergite | 2.00 / 2.00 | 3.00 |
| Anorpiment | 1.50 / 1.50 | 3.32 |
| Arakiite | 3.00 / 4.00 | |
| Ardennite-(As) | 6.00 / 7.00 | 3.62 |
| Ardennite-(V) | 6.00 / 7.00 | 3.55 |
| Argentobaumhauerite | 3.00 / 3.00 | 5.31 |
| Argentoliveingite | ||
| Argentotennantite | 3.50 / 3.50 | 5.00 |
| Arhbarite | ||
| Armangite | 4.00 / 4.00 | 4.43 |
| Arsenbrackebuschite | 4.50 / 4.50 | 6.54 |
| Arsendescloizite | 4.00 / 4.00 | 6.57 |
| Arsenic | 3.50 / 3.50 | 5.70 |
| Arseniopleite | 3.50 / 3.50 | 4.22 |
| Arseniosiderite | 1.50 / 1.50 | 3.50 |
| Arsenoclasite | 5.00 / 6.00 | 4.00 |
| Arsenocrandallite | 5.50 / 5.50 | 3.25 |
| Arsenoflorencite-(Ce) | 3.50 / 3.50 | 4.10 |
| Arsenoflorencite-(La) | 3.50 / 3.50 | 4.10 |
| Arsenoflorencite-(Nd) | 3.50 / 3.50 | 4.05 |
| Arsenogorceixite | 4.00 / 4.00 | 3.65 |
| Arsenogoyazite | 4.00 / 4.00 | 3.33 |
| Arsenohauchecornite | 5.50 / 5.50 | 6.35 |
| Arsenohopeite | 3.00 / 3.00 | |
| Arsenolamprite | 2.00 / 2.00 | 5.30 |
| Arsenolite | 1.50 / 1.50 | 3.70 |
| Arsenopalladinite | 4.00 / 4.00 | 10.40 |
| Arsenopyrite | 5.00 / 5.00 | 6.07 |
| Arsenovanmeersscheite | ||
| Arsenowagnerite | ||
| Arsenowaylandite | 4.00 / 5.00 | |
| Arsenpolybasite | 3.00 / 3.00 | 6.18 |
| Arsenquatrandorite | ||
| Arsentsumebite | 4.00 / 5.00 | 6.46 |
| Arsenuranospathite | 2.00 / 2.00 | 2.54 |
| Arsenuranylite | 2.50 / 2.50 | 4.25 |
| Arsiccioite | 2.00 / 2.50 | 5.99 |
| Arsmirandite | ||
| Arthurite | 3.00 / 4.00 | 3.02 |
| Asbecasite | 6.50 / 7.00 | 3.70 |
| Asselbornite | 3.00 / 3.00 | |
| Atelestite | 4.50 / 5.00 | 6.82 |
| Atheneite | 5.00 / 5.00 | 10.20 |
| Attikaite | 2.00 / 2.50 | 3.20 |
| Auriacusite | 3.00 / 3.00 | 4.45 |
| Austinite | 4.00 / 4.50 | 4.13 |
| Babánekite | ||
| Barahonaite-(Al) | ||
| Barahonaite-(Fe) | 3.03 | |
| Barikaite | 5.34 | |
| Bariopharmacoalumite | 3.50 / 3.50 | 2.58 |
| Bariopharmacosiderite | 3.00 / 3.00 | 3.07 |
| Barium-zinc alumopharmocosiderite | 2.50 / 2.50 | |
| Barrotite | ||
| Baumhauerite | 3.00 / 3.00 | 5.33 |
| Baumhauerite II | 3.00 / 3.00 | 5.00 |
| Baumstarkite | 2.50 / 2.50 | 5.33 |
| Bayldonite | 4.50 / 4.50 | 5.50 |
| Bearsite | 1.80 | |
| Bendadaite | ||
| Benleonardite | 3.50 / 3.50 | 7.00 |
| Bergslagite | 5.00 / 5.00 | 3.40 |
| Bernardite | 2.00 / 2.00 | 4.50 |
| Bernarlottiite | ||
| Berzeliite | 4.50 / 5.00 | 4.08 |
| Betpakdalite-CaCa | 3.00 / 3.00 | 2.91 |
| Betpakdalite-CaMg | 3.50 / 3.50 | 2.94 |
| Betpakdalite-NaCa | 2.89 | |
| Betpakdalite-NaNa | 3.00 / 3.00 | 2.88 |
| Bettertonite | 2.02 | |
| Beudantite | 4.00 / 4.00 | 4.10 |
| Biehlite | 1.00 / 1.50 | |
| Billingsleyite | 2.50 / 2.50 | 5.90 |
| Bonazziite | 2.50 / 2.50 | 3.54 |
| Borisenkoite | ||
| Borishanskiite | 4.00 / 4.00 | 10.00 |
| Boscardinite | 3.00 | |
| Bouazzerite | 2.81 | |
| Braccoite | 3.56 | |
| Bradaczekite |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





