List of elements
»
Actinium
»
Aluminum
»
Antimony
»
Argon
»
Arsenic
»
astatine
»
Barium
»
Bismuth
»
bohrium
»
Boron
»
Bromine
»
Cadmium
»
Calcium
»
Carbon
»
Cerium
»
cesium
»
Chlorine
»
Chromium
»
Cobalt
»
Copper
»
Curium
»
dubnium
»
Erbium
»
Europium
»
fermium
»
Fluorine
»
francium
»
Gallium
»
Gold
»
Hafnium
»
hassium
»
Helium
»
holmium
»
Hydrogen
»
Indium
»
Iodine
»
Iridium
»
Iron
»
Krypton
»
Lead
»
Lithium
»
lutetium
»
Mercury
»
Neon
»
Nickel
»
Nihonium
»
Niobium
»
Nitrogen
»
nobelium
»
Osmium
»
Oxygen
»
Platinum
»
Polonium
»
Radium
»
Radon
»
Rhenium
»
rhodium
»
Rubidium
»
Samarium
»
scandium
»
Selenium
»
Silicon
»
Silver
»
Sodium
»
Sulfur
»
Tantalum
»
Terbium
»
Thallium
»
Thorium
»
Thulium
»
Tin
»
Titanium
»
Tungsten
»
Uranium
»
Vanadium
»
Xenon
»
Yttrium
»
Zinc
Ag - Silver - TRANSITION METAL
Silver (chemical symbol Ag) is a chemical element that belongs to the transition metal family of the periodic table of elements. It has the atomic number 47 and is represented by an atom that has 47 protons and 47 electrons arranged around its nucleus. silver is a lustrous gray-white metal that occurs as a dense, lightweight solid. It is very ductile and malleable, and is characterized by its high thermal and electrical conductivity.
Most of the properties of silver are due to its strong bonds between its atoms. It is also recognized for its chemical stability, which means that it is not very sensitive to external chemical changes such as heat, humidity and oxidation. It can also withstand extreme temperatures and is not easily affected by bentonite or grease.
silver is a very noble metal that has been used to make jewelry, coins, and decorative items for thousands of years. It is also used to make mirrors, photovoltaic cells and electrical contacts, as it is extremely conductive. It is also used in medical devices and food industry technologies, as it is non-toxic and non-corrosive.
Most of the properties of silver are due to its strong bonds between its atoms. It is also recognized for its chemical stability, which means that it is not very sensitive to external chemical changes such as heat, humidity and oxidation. It can also withstand extreme temperatures and is not easily affected by bentonite or grease.
silver is a very noble metal that has been used to make jewelry, coins, and decorative items for thousands of years. It is also used to make mirrors, photovoltaic cells and electrical contacts, as it is extremely conductive. It is also used in medical devices and food industry technologies, as it is non-toxic and non-corrosive.
Synthetic
Radioactive
Liquid
Gaseous
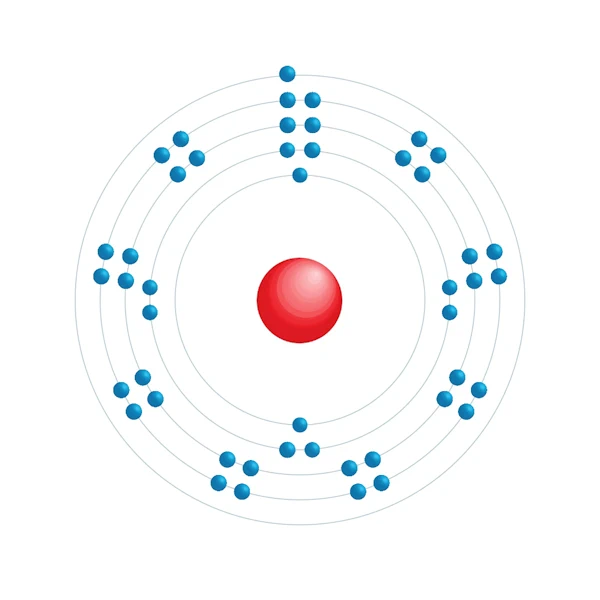
Electronic configuration diagram
| Name | Silver |
| Number | 47 |
| Atomic | 107.8682 |
| Symbol | Ag |
| Fusion | 961.9 |
| Boiling | 2212 |
| Density | 10.501 |
| Period | 5 |
| Group | 11 |
| Discovery | 0 Prehistoric |
| Abundance | 0.075 |
| Radius | 1.8 |
| Electronegativity | 1.93 |
| Ionization | 7.5762 |
| Number of isotopes | 27 |
| Electronic configuration | [Kr] 4d10 5s1 |
| Oxidation states | 1,2,3 |
| Electron by energy level | 2,8,18,18,1 |
| Mineral | Hardness | Density |
| Acanthite | 2.00 / 2.50 | 7.20 |
| Agmantinite | ||
| Aguilarite | 2.00 / 3.00 | 7.40 |
| Alburnite | 4.00 / 4.00 | 7.83 |
| Allargentum | 4.00 / 4.00 | 10.00 |
| Andorite IV | 3.50 / 3.50 | 5.00 |
| Andorite VI | 3.50 / 3.50 | 5.00 |
| Andreadiniite | ||
| Ángelaite | ||
| Aramayoite | 2.50 / 2.50 | 5.60 |
| Arcubisite | ||
| Argentobaumhauerite | 3.00 / 3.00 | 5.31 |
| Argentojarosite | 3.50 / 4.50 | 3.66 |
| Argentoliveingite | ||
| Argentopentlandite | 3.50 / 3.50 | 4.00 |
| Argentopyrite | 3.50 / 4.00 | 4.25 |
| Argentotennantite | 3.50 / 3.50 | 5.00 |
| Argentotetrahedrite | ||
| Argyrodite | 2.50 / 2.50 | 6.10 |
| Arsenpolybasite | 3.00 / 3.00 | 6.18 |
| Arsenquatrandorite | ||
| Arsiccioite | 2.00 / 2.50 | 5.99 |
| Aurorite | 2.00 / 3.00 | 3.80 |
| Balkanite | 3.50 / 3.50 | 6.32 |
| Barikaite | 5.34 | |
| Baumstarkite | 2.50 / 2.50 | 5.33 |
| Benjaminite | 3.50 / 3.50 | 6.70 |
| Benleonardite | 3.50 / 3.50 | 7.00 |
| Berryite | 3.50 / 3.50 | 6.70 |
| Bezsmertnovite | 4.50 / 4.50 | 16.30 |
| Bideauxite | 3.00 / 3.00 | 6.27 |
| Billingsleyite | 2.50 / 2.50 | 5.90 |
| Bohdanowiczite | 3.00 / 3.00 | 7.87 |
| Boleite | 3.00 / 3.50 | 4.80 |
| Borodaevite | 3.50 / 3.50 | 7.90 |
| Bromargyrite | 1.50 / 2.00 | 5.80 |
| Cameronite | 3.50 / 4.00 | 7.00 |
| Canfieldite | 2.50 / 2.50 | 6.28 |
| Capgaronnite | 6.19 | |
| Carducciite | 2.50 / 3.00 | 5.56 |
| Catamarcaite | 3.50 / 3.50 | 4.89 |
| Cervelleite | 1.50 / 1.50 | 8.00 |
| Chalcothallite | 2.00 / 2.50 | 6.60 |
| Chenguodaite | 2.00 / 3.00 | 6.85 |
| Chlorargyrite | 1.00 / 1.50 | 5.55 |
| Chrisstanleyite | 5.00 / 5.00 | 8.31 |
| Clino-oscarkempffite | ||
| Coiraite | 2.00 / 2.00 | 5.92 |
| Coldwellite | 9.90 | |
| Criddleite | 3.00 / 3.50 | 6.00 |
| Crookesite | 2.50 / 3.00 | 6.90 |
| Cuboargyrite | 3.00 / 3.00 | 5.33 |
| Cuprobismutite | 6.47 | |
| Cupromakopavonite | ||
| Cupromakovickyite | 4.00 / 4.50 | 6.78 |
| Cupropavonite | 2.00 / 2.00 | 7.00 |
| Cupropearceite | ||
| Cupropolybasite | 3.00 / 3.50 | 6.31 |
| Danielsite | 2.00 / 2.50 | 6.00 |
| Dantopaite | 3.50 / 3.50 | 6.74 |
| Debattistiite | ||
| Dervillite | 1.00 / 1.50 | 5.00 |
| Diaphorite | 2.50 / 2.50 | 6.00 |
| Dyscrasite | 3.50 / 4.00 | 9.40 |
| Eckerite | 2.50 / 3.00 | |
| Ecrinsite | ||
| Electrum | ||
| Empressite | 3.50 / 3.50 | 7.50 |
| Erzwiesite | ||
| Eskimoite | 4.00 / 4.00 | 7.10 |
| Eucairite | 2.50 / 2.50 | 7.60 |
| Eugenite | 2.50 / 3.00 | 10.75 |
| Ferdowsiite | 2.50 / 3.00 | 5.30 |
| Fettelite | 3.50 / 4.00 | 6.29 |
| Fischesserite | 2.00 / 2.50 | 9.05 |
| Fizélyite | 2.00 / 2.00 | 5.56 |
| Freibergite | 3.50 / 4.00 | 4.85 |
| Freieslebenite | 2.50 / 2.50 | 6.20 |
| Furutobeite | 3.00 / 3.00 | 6.74 |
| Gabrielite | 1.50 / 2.00 | 5.38 |
| Geffroyite | 2.50 / 2.50 | 5.39 |
| Giessenite | 2.50 / 2.50 | 7.45 |
| Giraudite | 3.50 / 4.00 | 5.75 |
| Goldamalgam | 3.00 / 3.00 | 15.47 |
| Gustavite | 3.50 / 3.50 | 7.01 |
| Hatchite | 5.00 | |
| Henryite | 3.50 / 3.50 | 7.86 |
| Hessite | 1.50 / 2.00 | 7.20 |
| Heyrovskýite | 3.50 / 4.00 | 7.11 |
| Hocartite | 4.00 / 4.00 | 4.77 |
| Hunchunite | 3.50 / 3.50 | 16.00 |
| Iltisite | 6.59 | |
| Imiterite | 2.50 / 3.00 | 7.85 |
| Iodargyrite | 1.50 / 2.00 | 5.50 |
| Jagüéite | 5.00 / 5.00 | 8.01 |
| Jalpaite | 2.00 / 2.50 | 6.82 |
| Jasrouxite | ||
| Jonassonite | 2.50 / 3.00 | |
| Keutschite |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se





